溶瘤病毒疗法:进展与挑战
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 33
摘要
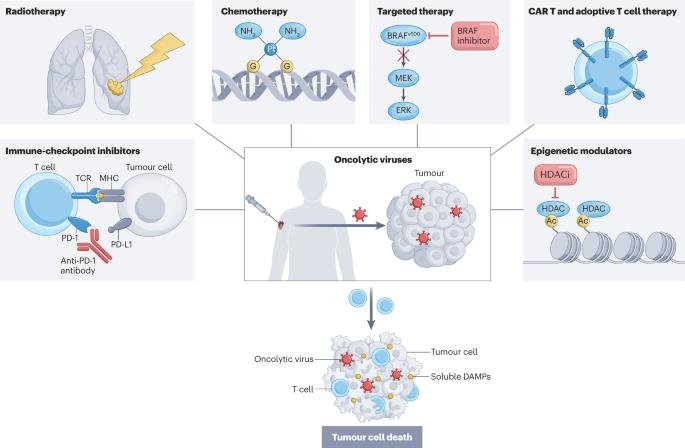
肿瘤溶解病毒(OVs)是一类新兴的癌症疗法,具有在肿瘤细胞中选择性复制、传递多种真核转基因有效载荷、诱导免疫原性细胞死亡和促进抗肿瘤免疫等优点,而且具有与其他癌症疗法基本不重叠的可耐受安全性。迄今为止,全球已有四种 OV 和一种非溶瘤病毒获准用于治疗癌症,但 talimogene laherparepvec(T-VEC)仍是唯一获得广泛批准的疗法。T-VEC适用于治疗初次手术后复发的黑色素瘤患者,于2015年首次获批。目前,有关接受T-VEC治疗的患者临床经验的数据不断增加,其他各种OVs治疗其他癌症的临床试验数据也在不断增加。尽管研究兴趣与日俱增,但要充分发挥这些药物在癌症患者中的治疗潜力,还需要更好地了解 OVs 的基础生物学和药理学。在本综述中,我们总结了现有的数据,并提供了在临床实践中优化使用 OVs 的指导,重点介绍了 T-VEC 的临床经验。我们介绍了目前正在进行临床开发的部分新型 OV 的数据,这些 OV 既可以作为单一疗法,也可以作为联合疗法的一部分。我们还讨论了迄今为止限制 OV 开发的一些临床前、临床和监管障碍。肿瘤溶解病毒(OV)提供了一种新型癌症治疗策略,其作用机制和毒性特征与传统疗法截然不同。迄今为止,全球已有四种 OV 进入临床应用,但只有 talimogene laherparepvec(T-VEC)进入了广泛的临床应用。在本《综述》中,作者介绍了迄今为止 T-VEC 的临床和监管经验,以及如何以此指导新型 OV 的开发。文中还讨论了一系列有可能在不久的将来应用于临床的新型 OV。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Therapy with oncolytic viruses: progress and challenges
Oncolytic viruses (OVs) are an emerging class of cancer therapeutics that offer the benefits of selective replication in tumour cells, delivery of multiple eukaryotic transgene payloads, induction of immunogenic cell death and promotion of antitumour immunity, and a tolerable safety profile that largely does not overlap with that of other cancer therapeutics. To date, four OVs and one non-oncolytic virus have been approved for the treatment of cancer globally although talimogene laherparepvec (T-VEC) remains the only widely approved therapy. T-VEC is indicated for the treatment of patients with recurrent melanoma after initial surgery and was initially approved in 2015. An expanding body of data on the clinical experience of patients receiving T-VEC is now becoming available as are data from clinical trials of various other OVs in a range of other cancers. Despite increasing research interest, a better understanding of the underlying biology and pharmacology of OVs is needed to enable the full therapeutic potential of these agents in patients with cancer. In this Review, we summarize the available data and provide guidance on optimizing the use of OVs in clinical practice, with a focus on the clinical experience with T-VEC. We describe data on selected novel OVs that are currently in clinical development, either as monotherapies or as part of combination regimens. We also discuss some of the preclinical, clinical and regulatory hurdles that have thus far limited the development of OVs. Oncolytic viruses (OVs) provide a novel cancer treatment strategy, with a mechanism of action and toxicity profiles that are distinctly different to those of more traditional therapies. Thus far, four OVs have entered clinical use globally, yet only talimogene laherparepvec (T-VEC) has entered widespread clinical use. In this Review, the authors describe the clinical and regulatory experience with T-VEC thus far, and how this can guide the development of novel OVs. Discussions of a range of novel OVs with the potential for clinical implementation in the near future are also provided.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


