青霉素酰化酶在氨基酸N-苄氧羰基衍生物脱保护中的特异性。
IF 2
4区 生物学
Q4 CELL BIOLOGY
引用次数: 0
摘要
N-酰基化氨基酸衍生物中N-酰基结构的变化显著影响青霉素酰化酶在该系列底物上的识别和活性。然而,来自粪产碱杆菌和大肠杆菌的青霉素酰化酶能够在温和条件下去除氨基酸衍生物中的N-苄氧羰基保护基,而不使用有毒试剂。利用现代合理的酶设计方法可以提高青霉素酰化酶在制备性有机合成中的应用效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
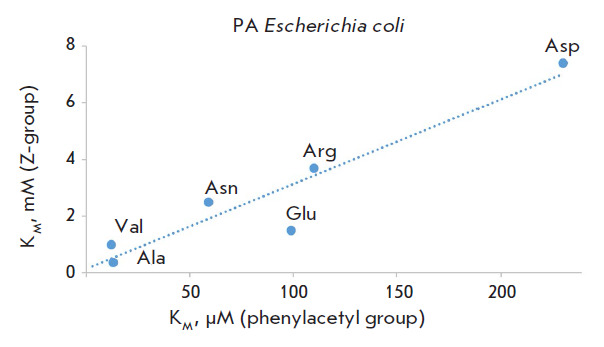
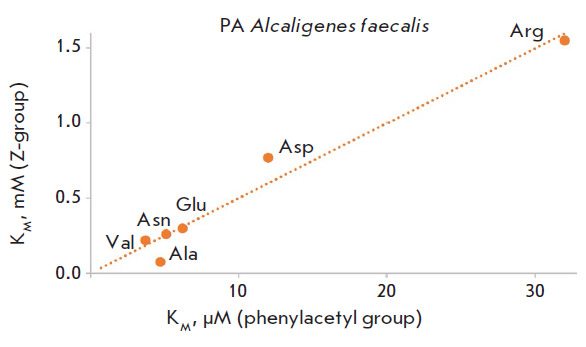
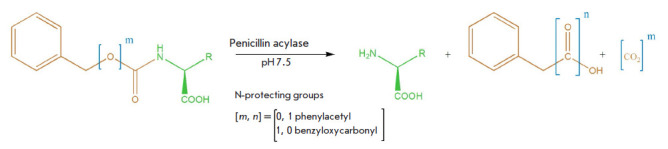
Specificity of Penicillin Acylases in Deprotection of N-Benzyloxycarbonyl Derivatives of Amino Acids.
Changes in the structure of the N-acyl group in N-acylated amino acid derivatives significantly affect both the recognition and activity of penicillin acylases on this series of substrates. However, penicillin acylases from both Alcaligenes faecalis and Escherichia coli are capable of removing the N-benzyloxycarbonyl protecting group in amino acid derivatives under mild conditions without the use of toxic reagents. Efficiency in using penicillin acylases in preparative organic synthesis can be improved by utilizing modern rational enzyme design methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Naturae
农林科学-林学
CiteScore
3.50
自引率
5.00%
发文量
0
审稿时长
>12 weeks
期刊介绍:
Acta Naturae is an international journal on life sciences based in Moscow, Russia.
Our goal is to present scientific work and discovery in molecular biology, biochemistry, biomedical disciplines and biotechnology. These fields represent the most important priorities for the research and engineering development both in Russia and worldwide. Acta Naturae is also a periodical for those who are curious in various aspects of biotechnological business, innovations in pharmaceutical areas, intellectual property protection and social consequences of scientific progress. The journal publishes analytical industrial surveys focused on the development of different spheres of modern life science and technology.
Being a radically new and totally unique journal in Russia, Acta Naturae is useful to both representatives of fundamental research and experts in applied sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


