病原体模拟聚合物纳米颗粒诱导 T 辅助-17 细胞介导的抗肿瘤免疫力
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 8
摘要
癌症免疫疗法的有效性受到免疫抑制性肿瘤微环境的阻碍,效应 T 细胞和自然杀伤细胞很少浸润肿瘤微环境。在感染和自身免疫性疾病中,效应免疫细胞的招募和激活是由促炎性T辅助细胞17(TH17)协调的。在这里,我们展示了显示甘露聚糖(微生物细胞壁中一种能激活TH17细胞的多糖)的病原体模拟空心纳米粒子,它能限制调节性T细胞的数量,并诱导TH17细胞介导的抗肿瘤反应。纳米颗粒能激活树突状细胞中的模式识别受体 Dectin-2 和 Toll 样受体 4,并促进 CD4+ T 细胞分化为 TH17 表型。在小鼠体内瘤内给药纳米粒子可降低肿瘤中调节性 T 细胞的比例,同时显著增加 TH17 细胞(以及 TH17 细胞相关细胞因子的水平)、CD8+ T 细胞、自然杀伤细胞和 M1 样巨噬细胞的比例。在多种小鼠模型中,针对共刺激受体 OX40 的激动抗体增强了效应细胞的抗肿瘤活性。诱导 TH17 细胞介导的免疫反应的纳米材料可能具有治疗潜力。瘤内注射显示甘露聚糖的空心纳米颗粒可降低肿瘤微环境中调节性T细胞的比例,并诱导由T辅助17细胞介导的抗肿瘤反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
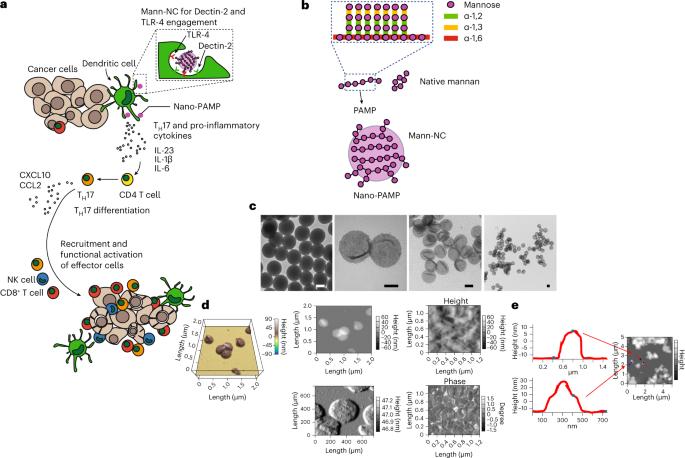
Induction of T-helper-17-cell-mediated anti-tumour immunity by pathogen-mimicking polymer nanoparticles
The effectivity of cancer immunotherapies is hindered by immunosuppressive tumour microenvironments that are poorly infiltrated by effector T cells and natural killer cells. In infection and autoimmune disease, the recruitment and activation of effector immune cells is coordinated by pro-inflammatory T helper 17 (TH17) cells. Here we show that pathogen-mimicking hollow nanoparticles displaying mannan (a polysaccharide that activates TH17 cells in microbial cell walls) limit the fraction of regulatory T cells and induce TH17-cell-mediated anti-tumour responses. The nanoparticles activate the pattern-recognition receptor Dectin-2 and Toll-like receptor 4 in dendritic cells, and promote the differentiation of CD4+ T cells into the TH17 phenotype. In mice, intra-tumoural administration of the nanoparticles decreased the fraction of regulatory T cells in the tumour while markedly increasing the fractions of TH17 cells (and the levels of TH17-cell-associated cytokines), CD8+ T cells, natural killer cells and M1-like macrophages. The anti-tumoural activity of the effector cells was amplified by an agonistic antibody against the co-stimulatory receptor OX40 in multiple mouse models. Nanomaterials that induce TH17-cell-mediated immune responses may have therapeutic potential. Intra-tumourally injected hollow nanoparticles displaying the polysaccharide mannan downregulate the fraction of regulatory T cells in the tumour microenvironment and induce anti-tumour responses mediated by T helper 17 cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


