运动单元的灵活神经控制
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 35
摘要
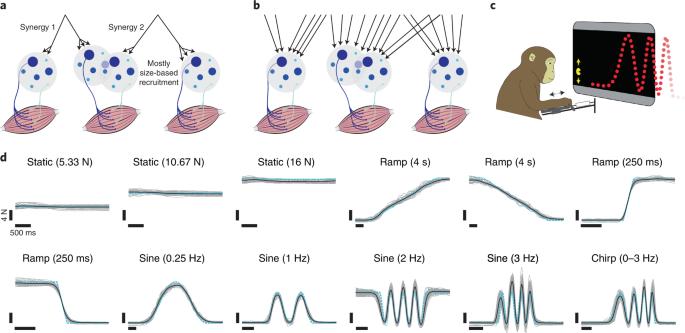
自主运动需要从大脑皮层到脊髓的通信,在脊髓中,专用的运动单元(MU)池激活每块肌肉。对运动单元功能的经典描述基于两个基本原则。首先,大脑皮层不能独立控制运动单元,而是为每个运动单元池提供共同的驱动力。其次,肌肉单位的招募方式是严格的,这在很大程度上符合海尼曼的大小原则。虽然这一范式得到了大量的经验支持,但要对其进行直接测试,需要在不同的力曲线上同时观察许多 MU。在这项研究中,我们开发了一种等长任务,即使在力量快速变化时,也能对猕猴的中枢神经单元进行稳定记录。令人惊讶的是,MU 的活动模式与行为有关,只有通过假定多重驱动才能准确描述。与灵活的降序控制相一致的是,对相邻皮层部位的微刺激会招募到不同的 MU。此外,皮层群体反应显示出足够的自由度,有可能施加精细的控制。因此,肌肉单位的活动可以灵活控制,以满足任务需求,而大脑皮层可能有助于提高这种能力。肌肉纤维具有不同的特性--例如,有慢抽动和快抽动之分。肌群由运动神经元激活。马歇尔等人发现,运动神经元的使用是灵活的,这可能使我们能够智能地使用适合每项任务的纤维。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Flexible neural control of motor units
Voluntary movement requires communication from cortex to the spinal cord, where a dedicated pool of motor units (MUs) activates each muscle. The canonical description of MU function rests upon two foundational tenets. First, cortex cannot control MUs independently but supplies each pool with a common drive. Second, MUs are recruited in a rigid fashion that largely accords with Henneman’s size principle. Although this paradigm has considerable empirical support, a direct test requires simultaneous observations of many MUs across diverse force profiles. In this study, we developed an isometric task that allowed stable MU recordings, in a rhesus macaque, even during rapidly changing forces. Patterns of MU activity were surprisingly behavior-dependent and could be accurately described only by assuming multiple drives. Consistent with flexible descending control, microstimulation of neighboring cortical sites recruited different MUs. Furthermore, the cortical population response displayed sufficient degrees of freedom to potentially exert fine-grained control. Thus, MU activity is flexibly controlled to meet task demands, and cortex may contribute to this ability. Muscle fibers have diverse properties—for example, slow and fast twitch. Groups of fibers are activated by motoneurons. Marshall et al. found that motoneurons are used flexibly, presumably allowing us to intelligently employ fibers suited to each task.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


