以肿瘤髓系细胞为治疗目标
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 16
摘要
髓系细胞在免疫抑制性肿瘤微环境中起着关键作用。来自单核细胞或中性粒细胞的肿瘤修饰髓系细胞(称为 "髓源性抑制细胞")和肿瘤相关巨噬细胞的聚集与不良预后以及对化疗和免疫检查点抑制剂等治疗的耐药性有关。遗憾的是,髓系细胞调节剂的大规模临床试验几乎没有取得成功,迄今为止,临床上仅使用了几种不同的策略来靶向抑制性髓系细胞。临床前和转化研究现已阐明了肿瘤微环境中不同髓系细胞亚群的特定功能,揭示了不同髓系细胞群在疾病进展和影响治疗反应中的特异性作用。为了提高髓系细胞靶向疗法的成功率,必须针对髓系细胞是耐药性主要驱动因素的肿瘤类型和患者亚群,并确定最有效的治疗方案和联合治疗伙伴。本综述将讨论我们能从第一代髓系调节剂的工作中学到什么,并重点介绍在模拟不同髓系细胞亚型的特异性作用方面的最新进展,最终为如何推动更成功的临床试验提供信息。肿瘤微环境中的髓样细胞对肿瘤的进展有很大影响,针对这些细胞的治疗一直是临床研究的重点。在这篇综述中,Barry 等人讨论了髓样细胞靶向疗法的临床前和临床数据,重点是了解特异性环境效应如何有助于设计这些药物的成功临床试验。本文章由计算机程序翻译,如有差异,请以英文原文为准。
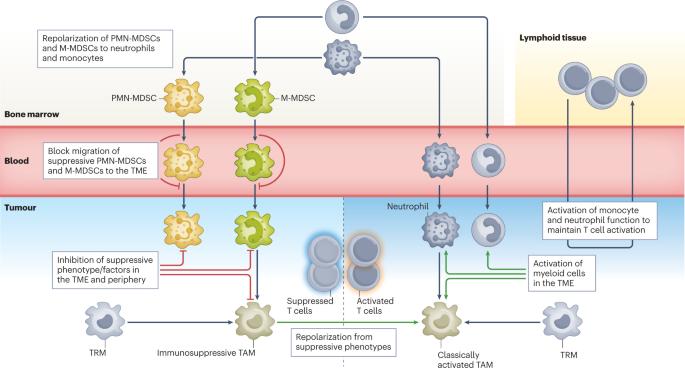
Therapeutic targeting of tumour myeloid cells
Myeloid cells are pivotal within the immunosuppressive tumour microenvironment. The accumulation of tumour-modified myeloid cells derived from monocytes or neutrophils — termed ‘myeloid-derived suppressor cells’ — and tumour-associated macrophages is associated with poor outcome and resistance to treatments such as chemotherapy and immune checkpoint inhibitors. Unfortunately, there has been little success in large-scale clinical trials of myeloid cell modulators, and only a few distinct strategies have been used to target suppressive myeloid cells clinically so far. Preclinical and translational studies have now elucidated specific functions for different myeloid cell subpopulations within the tumour microenvironment, revealing context-specific roles of different myeloid cell populations in disease progression and influencing response to therapy. To improve the success of myeloid cell-targeted therapies, it will be important to target tumour types and patient subsets in which myeloid cells represent the dominant driver of therapy resistance, as well as to determine the most efficacious treatment regimens and combination partners. This Review discusses what we can learn from work with the first generation of myeloid modulators and highlights recent developments in modelling context-specific roles for different myeloid cell subtypes, which can ultimately inform how to drive more successful clinical trials. Myeloid cells in the tumour microenvironment strongly influence tumour progression, and targeting these cells has been a key clinical focus. In this Review, Barry et al. discuss preclinical and clinical data on myeloid-targeting therapies, with a focus on how understanding context-specific effects might aid the design of successful clinical trials for these drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


