邻羟基亚胺酮在磺化盐和芳烃磺酰氯化物中的芳基化反应:异黄酮的一种途径
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 9
摘要
在这里,我们揭示了三种新的方法,用于直接和有效地合成3-芳基色酮后,邻羟基亚胺酮的芳基化由大量的实验稳定和易于使用的磺化盐和芳烃磺酰氯。这两种方法,即光介导的光氧化还原和亲电芳基化,都显示出良好的效率,并且可以以优异的收率制备3-芳基色素。这项工作展示了第一次描述的尝试,其中磺胺盐和芳烃磺酰氯被成功地用于构建色素杂环体系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
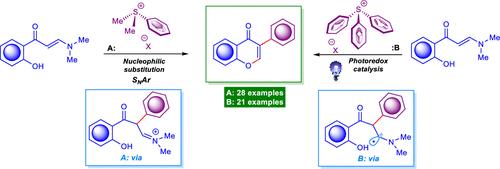
Arylation of ortho-Hydroxyarylenaminones by Sulfonium Salts and Arenesulfonyl Chlorides: An Access to Isoflavones
Herein we disclose three new methods for the straightforward and efficient synthesis of 3-arylchromones following the arylation of ortho-hydroxyarylenaminones by vast diversities of bench-stable and easy-to-use sulfonium salts and arenesulfonyl chlorides. Both developed methods, namely the light-mediated photoredox and electrophilic arylation, showed good efficiency, and are feasible for the preparation of 3-arylchromones in good-to-excellent yields. This work showcases the first described attempt where the sulfonium salts and arenesulfonyl chlorides were successfully utilized for the construction of the chromone heterocycle system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


