n -氯内酰胺的光化学重排:通过协同环收缩生成n -杂环的途径
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 35
摘要
我们报告了一种新的环收缩,允许n -氯内酰胺在光解时直接转化为相应的环收缩n -杂环。结果表明,多种n -氯内酰胺均发生重排,迁移碳取代量越大,产物收率越高。重要的是,迁移碳的立体化学在产物中是保守的。重新排列的产物被分离为氨基甲酸甲酯,收率从17%到58%不等,主要副产物是可回收的母体内酰胺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
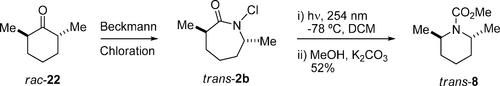
Photochemical Rearrangement of N-Chlorolactams: A Route to N-Heterocycles through Concerted Ring Contraction
We report a novel ring contraction allowing the direct conversion of N-chlorolactams to their corresponding ring-contraction N-heterocycles upon photolysis. Results show that the rearrangement occurs with a variety of N-chlorolactams and that the greater the substitution at the migrating carbon, the greater the yield of product. Importantly, stereochemistry at the migrating carbon is conserved in the product. Rearranged products were isolated as their methyl carbamates in yields varying from 17% to 58%, with the major side product being the recyclable parent lactam.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


