基因修饰CD7靶向异基因CAR-T细胞治疗复发/难治性CD7阳性血液系统恶性肿瘤的疗效增强:一项I期临床研究。
IF 28.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 32
摘要
嵌合抗原受体(CAR)-T细胞治疗T细胞恶性肿瘤面临重大挑战,包括CAR-T细胞之间的自相残杀和来自母细胞的产物污染。由健康供体T细胞产生的异基因CAR-T细胞可以提供现成的、无爆炸的治疗产品,但它们的应用可能会因移植物抗宿主病(GvHD)和宿主排斥而变得复杂。在这里,我们开发了健康供体来源的CD7靶向CAR-T细胞(RD13-01),该细胞具有基因修饰,可抵抗自相残杀、GvHD和异基因排斥反应,并增强抗肿瘤功能。一项I期临床试验(NCT04538599)招募了12名患者(11名患有T细胞白血病/淋巴瘤,1名患有表达CD7的急性髓系白血病)。所有患者都达到了预先设定的终点,11名患者进行了疗效评估。无剂量限制毒性、GvHD、免疫效应细胞相关神经毒性或严重细胞因子释放综合征(分级 ≥ 3) 观察到。输注后28天,81.8%的患者(9/11)表现出客观反应,完全反应率为63.6%(7/11,包括AML患者)。有反应的患者中有3例接受了异基因造血干细胞移植。中位随访10.5个月,4名患者仍处于完全缓解状态。在几名患者中观察到巨细胞病毒(CMV)和/或EB病毒(EBV)的再激活,其中一人死于EBV相关的弥漫性大B细胞淋巴瘤(DLBCL)。输注后检测到CD7阴性的正常T细胞的扩增。总之,我们首次报道了使用健康供体来源的CD7靶向异基因CAR-T细胞治疗CD7+血液系统恶性肿瘤的I期临床试验。我们的结果证明了RD13-01异基因CAR-T细胞对CD7+肿瘤的安全性和有效性令人鼓舞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
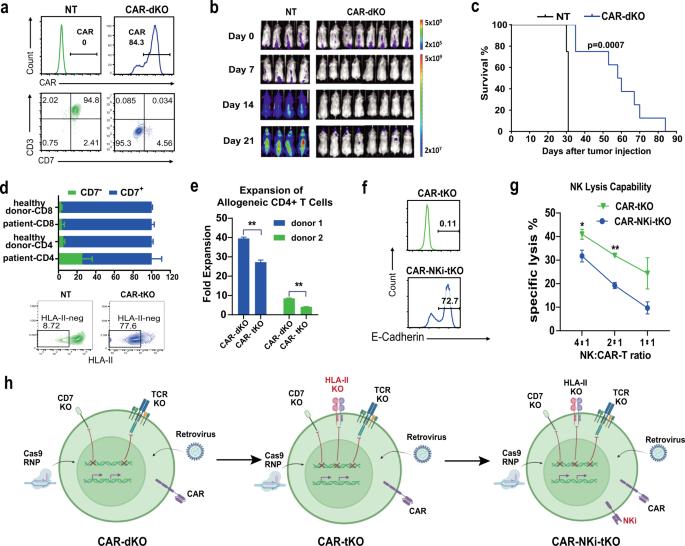
Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study
Chimeric antigen receptor (CAR)-T cell therapy against T cell malignancies faces major challenges including fratricide between CAR-T cells and product contamination from the blasts. Allogeneic CAR-T cells, generated from healthy donor T cells, can provide ready-to-use, blast-free therapeutic products, but their application could be complicated by graft-versus-host disease (GvHD) and host rejection. Here we developed healthy donor-derived, CD7-targeting CAR-T cells (RD13-01) with genetic modifications to resist fratricide, GvHD and allogeneic rejection, as well as to potentiate antitumor function. A phase I clinical trial (NCT04538599) was conducted with twelve patients recruited (eleven with T cell leukemia/lymphoma, and one with CD7-expressing acute myeloid leukemia). All patients achieved pre-set end points and eleven proceeded to efficacy evaluation. No dose-limiting toxicity, GvHD, immune effector cell-associated neurotoxicity or severe cytokine release syndrome (grade ≥ 3) were observed. 28 days post infusion, 81.8% of patients (9/11) showed objective responses and the complete response rate was 63.6% (7/11, including the patient with AML). 3 of the responding patients were bridged to allogeneic hematopoietic stem cell transplantation. With a median follow-up of 10.5 months, 4 patients remained in complete remission. Cytomegalovirus (CMV) and/or Epstein-Barr virus (EBV) reactivation was observed in several patients, and one died from EBV-associated diffuse large B-cell lymphoma (DLBCL). Expansion of CD7-negative normal T cells was detected post infusion. In summary, we present the first report of a Phase I clinical trial using healthy donor-derived CD7-targeting allogeneic CAR-T cells to treat CD7+ hematological malignancies. Our results demonstrated the encouraging safety and efficacy profiles of the RD13-01 allogeneic CAR-T cells for CD7+ tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


