苍术血清总生物活性的生物测定。
IF 1.4
Q3 Pharmacology, Toxicology and Pharmaceutics
Journal of Advanced Pharmaceutical Technology & Research
Pub Date : 2023-01-01
Epub Date: 2023-01-20
DOI:10.4103/japtr.japtr_431_22
引用次数: 2
摘要
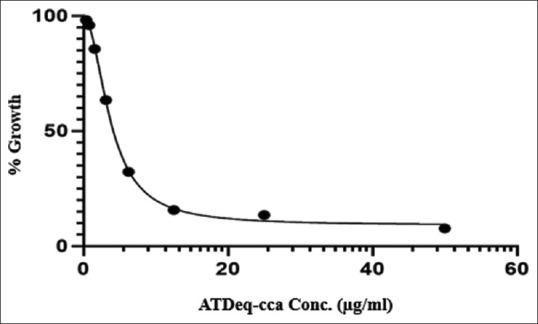
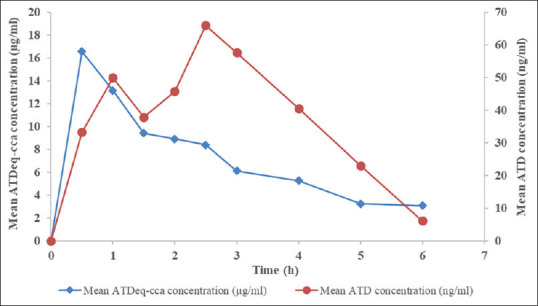
本研究旨在建立人体血清样品中苍术总生物活性的生物测定方法。使用3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑溴化物测定法评估对细菌生长的抑制作用(金黄色葡萄球菌ATCC 25923)。校准曲线(0、0.39、0.78、1.56、3.13、2.56和50ng/mμl)呈线性,相关系数>0.990。使用20ml血清样品,定量限(LOQ)为1.66mg/ml。所建立的生物测定方法符合生物分析法测定AL血清生物活性的标准。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bioassay for total serum bioactivity of Atractylodes lancea.
The study aimed to establish a bioassay for total bioactivity of Atractylodes lancea (AL) in human serum samples. Inhibition of bacterial growth (Staphylococcus aureus ATCC 25923) was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The calibration curve (0, 0.39, 0.78, 1.56, 3.13, 2.56, and 50 ng/μl) was linear with correlation coefficients >0.990. The limit of quantification (LOQ) was 1.66 mg/ml using 20-ml serum sample. The developed bioassay method meets the standard of the bioanalytical method for determination of serum bioactivity of AL.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Pharmaceutical Technology & Research
PHARMACOLOGY & PHARMACY-
CiteScore
2.00
自引率
7.10%
发文量
44
审稿时长
20 weeks
期刊介绍:
Journal of Advanced Pharmaceutical Technology & Research (JAPTR) is an Official Publication of Society of Pharmaceutical Education & Research™. It is an international journal published Quarterly. Journal of Advanced Pharmaceutical Technology & Research (JAPTR) is available in online and print version. It is a peer reviewed journal aiming to communicate high quality original research work, reviews, short communications, case report, Ethics Forum, Education Forum and Letter to editor that contribute significantly to further the scientific knowledge related to the field of Pharmacy i.e. Pharmaceutics, Pharmacology, Pharmacognosy, Pharmaceutical Chemistry. Articles with timely interest and newer research concepts will be given more preference.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


