熊果酸衍生物 UA232 通过诱导内质网应激和溶酶体功能紊乱促进肿瘤细胞凋亡
IF 1.4
4区 农林科学
Q3 AGRICULTURE, MULTIDISCIPLINARY
Irish Journal of Agricultural and Food Research
Pub Date : 2022-03-21
eCollection Date: 2022-01-01
DOI:10.7150/ijbs.67166
引用次数: 12
摘要
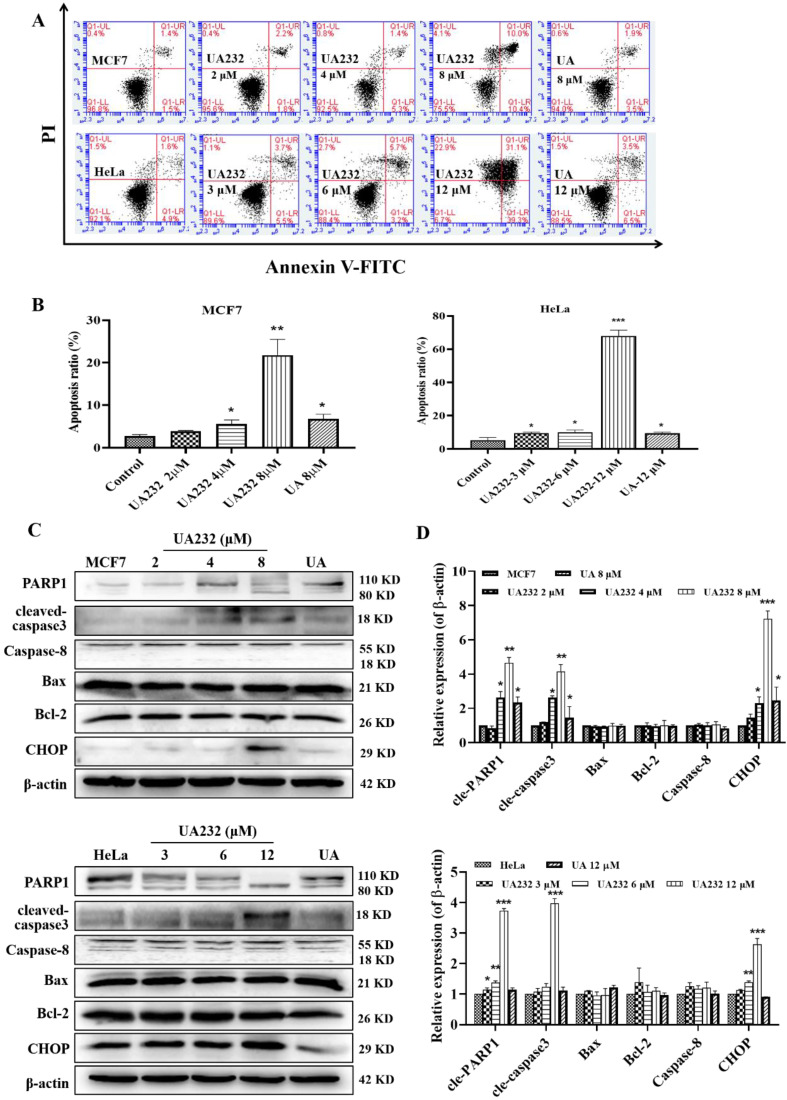
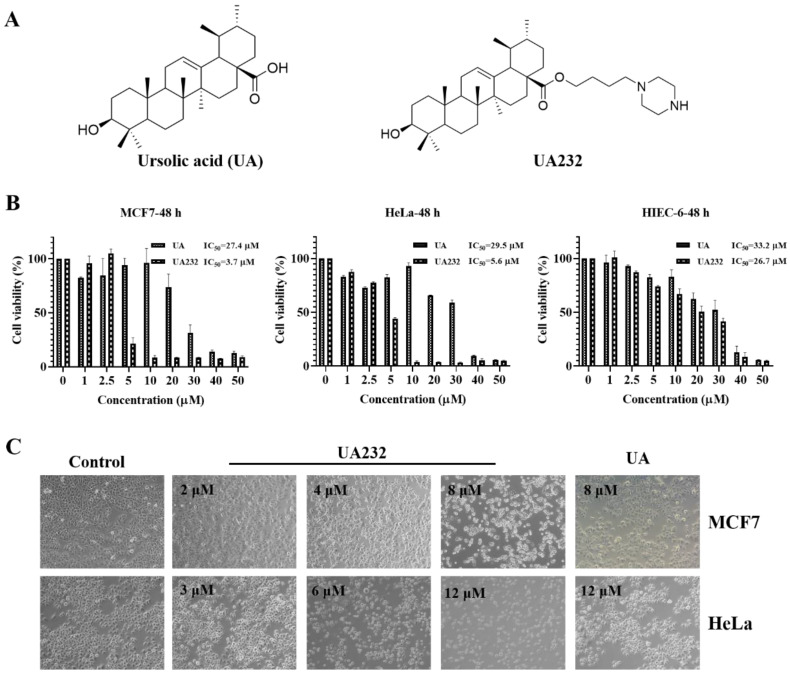
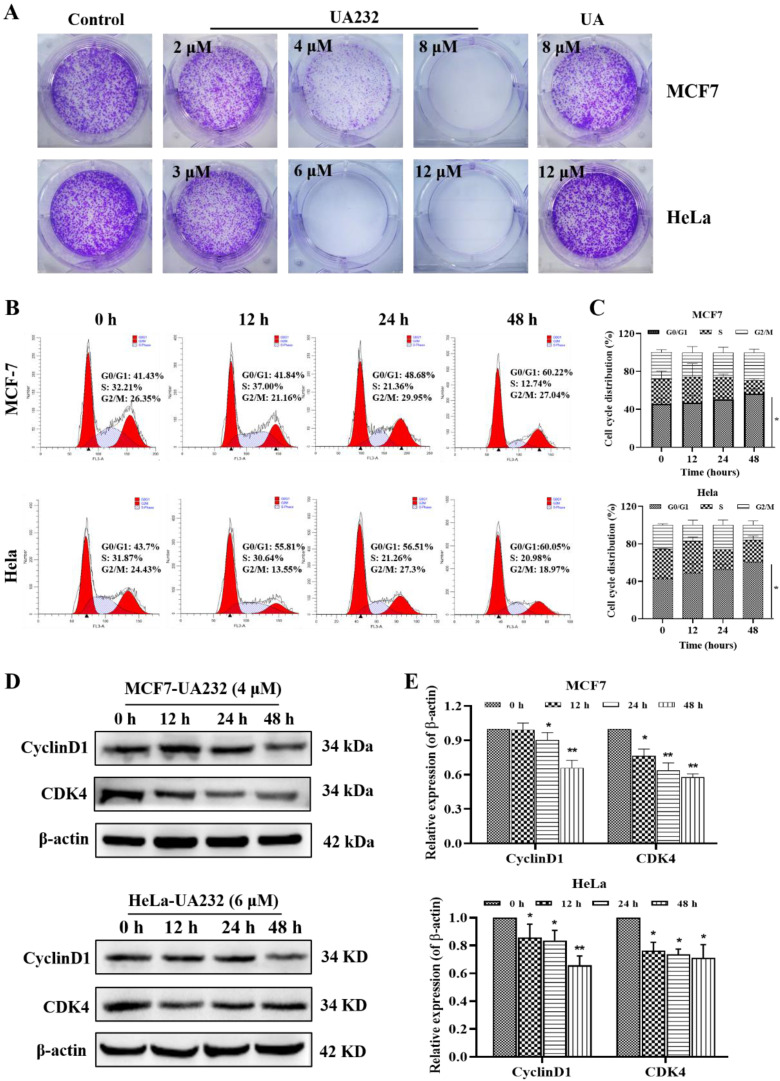
由于对药物和辐射的耐受性增强,开发新型抗癌药物迫在眉睫。在之前的研究中,我们对五环三萜类天然产物熊果酸(UA)进行了一系列结构改造,发现了一种具有更强抗肿瘤活性的衍生物 UA232。体外实验表明,UA232 可抑制人乳腺癌和宫颈癌细胞的增殖,诱导其 G0/G1 停滞,并促进其凋亡。机理研究发现,UA232通过蛋白激酶R样内质网激酶/激活转录因子4/C/EBP同源蛋白介导的内质网应激,促进细胞凋亡并诱导保护性自噬。此外,我们还发现 UA232 能诱导溶酶体生物生成,增加溶酶体膜通透性,促进溶酶体蛋白酶释放,并导致溶酶体依赖性细胞死亡。此外,UA232 还能抑制小鼠异种移植模型中的肿瘤生长。总之,我们的研究揭示了 UA232 通过同时引发内质网应激和溶酶体功能障碍,对乳腺癌和宫颈癌产生多重药理作用。因此,UA232 可能是一种治疗癌症的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ursolic Acid Derivative UA232 Promotes Tumor Cell Apoptosis by Inducing Endoplasmic Reticulum Stress and Lysosomal Dysfunction.
Due to increased drug and radiation tolerance, there is an urgent need to develop novel anticancer agents. In our previous study, we performed a series of structural modifications of ursolic acid (UA), a natural product of pentacyclic triterpenes, and found UA232, a derivative with stronger anti-tumor activity. In vitro experiments showed that UA232 inhibited proliferation, induced G0/G1 arrest, and promoted apoptosis in human breast cancer and cervical cancer cells. Mechanistic studies revealed that UA232 promoted apoptosis and induced protective autophagy via the protein kinase R-like endoplasmic reticulum kinase/activating transcription factor 4/C/EBP homologous protein-mediated endoplasmic reticulum stress. In addition, we also found that UA232 induced lysosomal biogenesis, increased lysosomal membrane permeability, promoted lysosomal protease release, and led to lysosome-dependent cell death. Furthermore, UA232 suppressed tumor growth in a mouse xenograft model. In conclusion, our study revealed that UA232 exerts multiple pharmacological effects against breast and cervical cancers by simultaneously triggering endoplasmic reticulum stress and lysosomal dysfunction. Thus, UA232 may be a promising drug candidate for cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
2.50
自引率
20.00%
发文量
23
审稿时长
>36 weeks
期刊介绍:
The Irish Journal of Agricultural and Food Research is a peer reviewed open access scientific journal published by Teagasc (Agriculture and Food Development Authority, Ireland). Manuscripts on any aspect of research of direct relevance to Irish agriculture and food production, including plant and animal sciences, food science, agri environmental science, soils, engineering, buildings, economics and sociology, will be considered for publication. The work must demonstrate novelty and relevance to the field of research. Papers published or offered for publication elsewhere will not be considered, but the publication of an abstract does not preclude the publication of the full paper in this journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


