新型喹唑啉类PI3K δ选择性抑制剂的发现和药理表征
IF 4
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 39
摘要
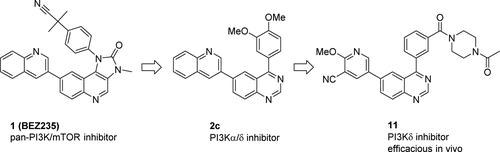
抑制脂质激酶PI3Kδ是治疗B和T细胞驱动的炎症性疾病的一个有希望的原理。使用支架解构-重建策略,我们确定了4-芳基喹唑啉,这些喹唑啉被优化成有效的PI3Kδ异构体选择性类似物,具有良好的药代动力学性质。通过化合物11,我们证明了生化PI3Kδ抑制可转化为异构体依赖性免疫细胞功能的调节(人、大鼠和小鼠)。在给大鼠口服化合物11后,近端PD标记物被抑制,在机械性斑块形成细胞试验中可以证明剂量依赖的功效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery and Pharmacological Characterization of Novel Quinazoline-Based PI3K Delta-Selective Inhibitors
Inhibition of the lipid kinase PI3Kδ is a promising principle to treat B and T cell driven inflammatory diseases. Using a scaffold deconstruction–reconstruction strategy, we identified 4-aryl quinazolines that were optimized into potent PI3Kδ isoform selective analogues with good pharmacokinetic properties. With compound 11, we illustrate that biochemical PI3Kδ inhibition translates into modulation of isoform-dependent immune cell function (human, rat, and mouse). After oral administration of compound 11 to rats, proximal PD markers are inhibited, and dose-dependent efficacy in a mechanistic plaque forming cell assay could be demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
ACS Medicinal Chemistry Letters
CHEMISTRY, MEDICINAL-
CiteScore
7.30
自引率
2.40%
发文量
328
审稿时长
1 months
期刊介绍:
ACS Medicinal Chemistry Letters is interested in receiving manuscripts that discuss various aspects of medicinal chemistry. The journal will publish studies that pertain to a broad range of subject matter, including compound design and optimization, biological evaluation, drug delivery, imaging agents, and pharmacology of both small and large bioactive molecules. Specific areas include but are not limited to:
Identification, synthesis, and optimization of lead biologically active molecules and drugs (small molecules and biologics)
Biological characterization of new molecular entities in the context of drug discovery
Computational, cheminformatics, and structural studies for the identification or SAR analysis of bioactive molecules, ligands and their targets, etc.
Novel and improved methodologies, including radiation biochemistry, with broad application to medicinal chemistry
Discovery technologies for biologically active molecules from both synthetic and natural (plant and other) sources
Pharmacokinetic/pharmacodynamic studies that address mechanisms underlying drug disposition and response
Pharmacogenetic and pharmacogenomic studies used to enhance drug design and the translation of medicinal chemistry into the clinic
Mechanistic drug metabolism and regulation of metabolic enzyme gene expression
Chemistry patents relevant to the medicinal chemistry field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


