闪锌矿-方铅矿在氯化钠溶液中的替代:热力学方法
IF 0.8
4区 地球科学
Q3 GEOLOGY
引用次数: 0
摘要
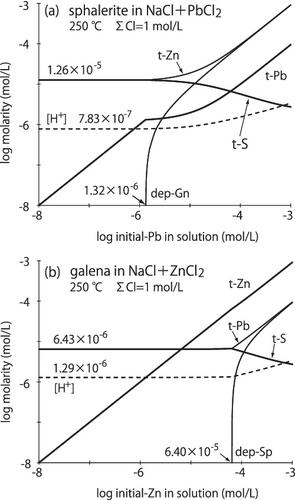
考虑组分的物质平衡(包括固相),在250℃、水饱和蒸汽压和初始Cl浓度为1 mol/L的平衡条件下,定量研究了闪锌矿-方铅矿对在氯化物溶液中的取代反应。含闪锌矿的NaCl+PbCl2溶液在无PbCl2或较低PbCl2浓度下溶解并释放出1.26 × 10−5 mol/L的总Zn和总S。当PbCl2浓度大于1.32 × 10−6 mol/L时,方铅矿作为替代物析出,表明闪锌矿具有捕获低浓度Pb的能力。当溶液中PbCl2浓度大于1.32 × 10−6 mol/L时,大部分Pb以方铅矿的形式沉积,并利用硫来源于固态闪锌矿的溶解,闪锌矿中的Zn相当于方铅矿的沉积量释放到溶液中。另一方面,在相同的环境条件下,含固体方铅矿的NaCl+ZnCl2溶液在无ZnCl2或较低ZnCl2浓度下溶解并释放出6.43 × 10−6 mol/L的总Pb和总S。当溶液中ZnCl2浓度为6.40 × 10−5 mol/L时,闪锌矿析出,说明方铅矿不能捕获低浓度的Zn。只有方铅矿存在时,锌会从热液沉积环境中流失。这些关系主要是由溶液中主要的金属氯化物或金属氢氧化物的反应控制的。闪锌矿对Pb有较好的清除作用,方铅矿对Zn没有清除作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sphalerite‐galena replacement in sodium chloride solution: A thermodynamic approach
Considering the material balances of the constituents including solid phases, replacement reaction of the sphalerite‐galena pair in chloride solution is examined quantitatively under equilibrium conditions of 250°C, water saturation vapor pressure, and initial Cl concentration of 1 mol/L. NaCl+PbCl2 solution with solid sphalerite, dissolves and releases both total Zn and total S of 1.26 × 10−5 mol/L into the solution under without or lower PbCl2 concentration. If the PbCl2 concentration is higher than 1.32 × 10−6 mol/L, precipitation of galena as replacement occurs, suggesting that sphalerite has an ability to trap a lower concentration of Pb. If PbCl2 concentration of the solution is higher than 1.32 × 10−6 mol/L, the majority of Pb deposited as galena with using sulfur originated from solid sphalerite dissolved, and the amount of Zn from sphalerite equivalent to the amount of galena deposited releases into the solution. On the other hand, NaCl+ZnCl2 solution with solid galena under the same environmental conditions, dissolves and releases both total Pb and total S of 6.43 × 10−6 mol/L into the solution under without or lower ZnCl2 concentration. Over the ZnCl2 concentration of 6.40 × 10−5 mol/L in the solution, precipitation of sphalerite occurs, indicating that galena cannot trap a low concentration of Zn. Zinc would drain away from the hydrothermal depositional environment under the presence of only galena. These relationships are controlled mainly by the reaction of predominant metal chloride or metal hydroxide species in the solution. Sphalerite is a good scavenger for Pb, but galena is not for Zn.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Resource Geology
地学-地质学
CiteScore
2.30
自引率
14.30%
发文量
18
审稿时长
12 months
期刊介绍:
Resource Geology is an international journal focusing on economic geology, geochemistry and environmental geology. Its purpose is to contribute to the promotion of earth sciences related to metallic and non-metallic mineral deposits mainly in Asia, Oceania and the Circum-Pacific region, although other parts of the world are also considered.
Launched in 1998 by the Society for Resource Geology, the journal is published quarterly in English, making it more accessible to the international geological community. The journal publishes high quality papers of interest to those engaged in research and exploration of mineral deposits.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


