大肠杆菌腺苷酸激酶的1H,13C,15N骨架共振定位
IF 0.8
4区 生物学
Q4 BIOPHYSICS
引用次数: 0
摘要
腺苷酸激酶可逆地催化ATP加AMP转化为两个ADP。这种重要的催化剂存在于每个细胞中,大肠杆菌蛋白经常被用作模型酶。我们的目的是使用大肠杆菌酶来了解干蛋白的结构和保护作用。在此,我们报道了其C77S变体的表达、纯化、稳态测定、NMR条件和1H、13C、15N骨架共振NMR归属。这些数据也将帮助其他人利用这种原型酶。本文章由计算机程序翻译,如有差异,请以英文原文为准。
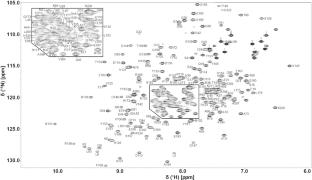
1H, 13C, 15N backbone resonance assignment of Escherichia coli adenylate kinase
Adenylate kinase reversibly catalyzes the conversion of ATP plus AMP to two ADPs. This essential catalyst is present in every cell, and the Escherichia coli protein is often employed as a model enzyme. Our aim is to use the E. coli enzyme to understand dry protein structure and protection. Here, we report the expression, purification, steady-state assay, NMR conditions and 1H, 13C, 15N backbone resonance NMR assignments of its C77S variant. These data will also help others utilize this prototypical enzyme.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


