局部的高浓度电解质通过胶束状结构得到更多的局部。
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电池中的液体电解质通常被视为宏观均匀的离子传输介质,尽管其具有复杂的化学组成和原子溶剂化结构,但在微观结构特征方面留下了知识空白。在这里,我们揭示了局部高浓度电解质中独特的胶束状结构,其中溶剂充当稀释剂中不溶性盐之间的表面活性剂。溶剂与稀释剂的混溶性和盐的同时溶解性导致具有涂抹界面的胶束状结构和在盐-溶剂簇的中心增加的盐浓度,这扩展了盐的溶解度。这些混合混溶性效应具有温度依赖性,其中典型的局部高浓度电解质在室温附近的局部簇盐浓度中达到峰值,并用于在Li金属阳极上形成稳定的固体电解质界面。这些发现为预测稳定的三元相图提供了指导,并将电解质微观结构与电解质配方和固体电解质界面的形成方案联系起来,以增强电池的可循环性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
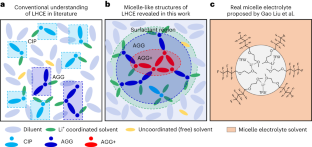
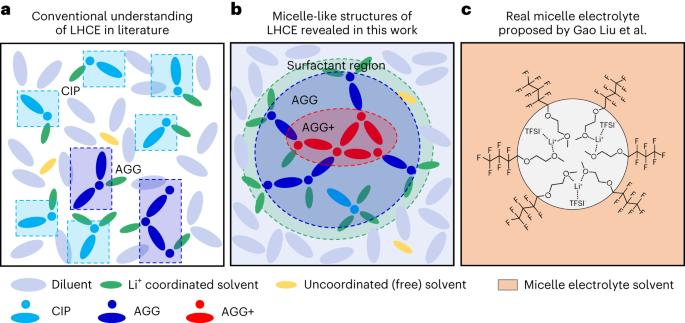
Localized high-concentration electrolytes get more localized through micelle-like structures
Liquid electrolytes in batteries are typically treated as macroscopically homogeneous ionic transport media despite having a complex chemical composition and atomistic solvation structures, leaving a knowledge gap of the microstructural characteristics. Here, we reveal a unique micelle-like structure in a localized high-concentration electrolyte, in which the solvent acts as a surfactant between an insoluble salt in a diluent. The miscibility of the solvent with the diluent and simultaneous solubility of the salt results in a micelle-like structure with a smeared interface and an increased salt concentration at the centre of the salt–solvent clusters that extends the salt solubility. These intermingling miscibility effects have temperature dependencies, wherein a typical localized high-concentration electrolyte peaks in localized cluster salt concentration near room temperature and is used to form a stable solid–electrolyte interphase on a Li metal anode. These findings serve as a guide to predicting a stable ternary phase diagram and connecting the electrolyte microstructure with electrolyte formulation and formation protocols of solid–electrolyte interphases for enhanced battery cyclability. Liquid electrolytes in batteries are considered to be macroscopically homogeneous ionic transport media despite having a complex chemical composition and atomistic solvation structures. A micelle-like structure in a localized high-concentration electrolyte for which the solvent acts as a surfactant is reported.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


