癌症新辅助治疗后病理反应与生存率的关系。
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
新辅助免疫疗法加化疗可提高无事件生存率(EFS)和病理完全反应(pCR,原发肿瘤[PT]+淋巴结[LNs]中0%残余活瘤[RVT]),并被批准用于治疗可切除的癌症。新辅助治疗后的病理反应评估是晚期疾病放射学反应的潜在类似物。然而,超过pCR的%RVT阈值和主要病理反应(≤10%RVT)尚未被探索。在随机3期CheckMate 816试验(NCT02998528)中前瞻性评估了病理反应,该试验评估了可切除肺癌癌症患者的新辅助nivolumab(抗PD-1)加化疗。使用泛肿瘤评分系统对PT和LN的RVT、回归和坏死进行量化(0%-100%),并在预先指定的探索性分析中测试其与EFS的相关性。无论LN是否参与,EFS改善0%,而RVT-PT改善>0%(HR = 0.18)。RVT-PT预测nivolumab加化疗的EFS(AUC = 0.74);RVT为0%-5%、>5%-30%、>30%-80%和>80%的患者的2年EFS发生率分别为90%、60%、57%和39%。每1%的RVT与EFS的0.017 HR增加相关。合并PT+LNs的病理反应有助于区分结果。与放射学反应和ctDNA清除率相比,%RVT最接近EFS。这些发现支持病理反应作为一种新兴的生存替代品。有必要进一步评估癌症和其他肿瘤类型中%RVT的全谱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
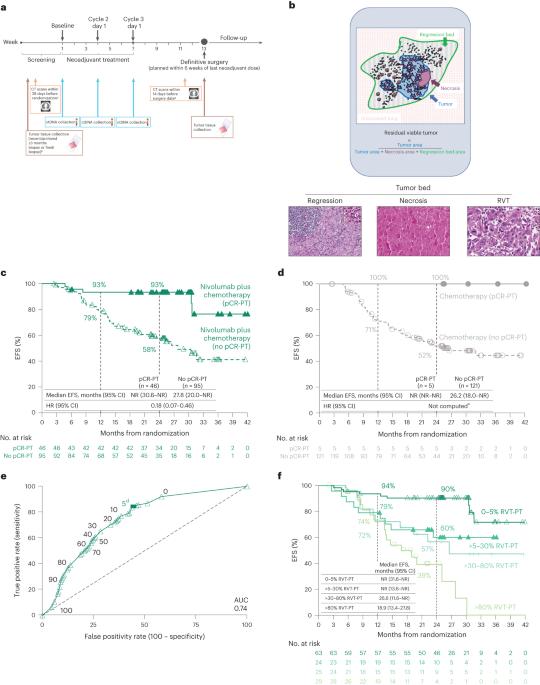
Association between pathologic response and survival after neoadjuvant therapy in lung cancer
Neoadjuvant immunotherapy plus chemotherapy improves event-free survival (EFS) and pathologic complete response (0% residual viable tumor (RVT) in primary tumor (PT) and lymph nodes (LNs)), and is approved for treatment of resectable lung cancer. Pathologic response assessment after neoadjuvant therapy is the potential analog to radiographic response for advanced disease. However, %RVT thresholds beyond pathologic complete response and major pathologic response (≤10% RVT) have not been explored. Pathologic response was prospectively assessed in the randomized, phase 3 CheckMate 816 trial (NCT02998528), which evaluated neoadjuvant nivolumab (anti-programmed death protein 1) plus chemotherapy in patients with resectable lung cancer. RVT, regression and necrosis were quantified (0–100%) in PT and LNs using a pan-tumor scoring system and tested for association with EFS in a prespecified exploratory analysis. Regardless of LN involvement, EFS improved with 0% versus >0% RVT-PT (hazard ratio = 0.18). RVT-PT predicted EFS for nivolumab plus chemotherapy (area under the curve = 0.74); 2-year EFS rates were 90%, 60%, 57% and 39% for patients with 0–5%, >5–30%, >30–80% and >80% RVT, respectively. Each 1% RVT associated with a 0.017 hazard ratio increase for EFS. Combining pathologic response from PT and LNs helped differentiate outcomes. When compared with radiographic response and circulating tumor DNA clearance, %RVT best approximated EFS. These findings support pathologic response as an emerging survival surrogate. Further assessment of the full spectrum of %RVT in lung cancer and other tumor types is warranted. ClinicalTrials.gov registration: NCT02998528 . Analysis of the phase 3 CheckMate 816 trial shows that the depth of pathologic response as assessed by percent residual viable tumor is correlated with event-free survival following neoadjuvant immunotherapy plus chemotherapy, supporting pathologic response as a biomarker of survival.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


