由(R)-甘二醇快速高效合成抗结核药Pretomanid
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
本文报道了一种用简单的化学反应、温和的反应条件和容易获得的起始材料有效的克级合成抗结核药普雷托马尼德。考察了四种不同保护基团对甘二醇部分的技术可行性和抑制副反应的能力。从容易获得的保护(R)-糖基醇和2-溴-4-硝基- 1h -咪唑开始,可以在线性三步合成中制备pretomanid,分离收率高达40%。与迄今为止报道的大多数合成相反,脱保护和环化是在一锅方式下进行的,没有任何危险步骤或起始材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
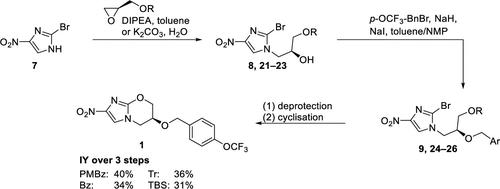
Short and Efficient Synthesis of the Antituberculosis Agent Pretomanid from (R)-Glycidol
An efficient gram-scale synthesis of the antituberculosis agent pretomanid using straightforward chemistry, mild reaction conditions, and readily available starting materials is reported. Four different protecting groups on the glycidol moiety were investigated for their technical feasibility and ability to suppress side reactions. Starting from readily available protected (R)-glycidols and 2-bromo-4-nitro-1H-imidazole, pretomanid could be prepared in a linear three-step synthesis in up to 40% isolated yield. In contrast to most syntheses reported so far, deprotection and cyclization were performed in a one-pot fashion without any hazardous steps or starting materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


