过氧化氢在气体爆轰中的双重行为
摘要
本文描述了过氧化氢加入到氢-空气起爆混合物中所观察到的双重行为。采用一维ZND计算方法,对\(\text {H}_{2}\)空气混合物中加入过氧化氢对\(\text {NO}_{x}\)排放和临界爆轰参数的影响进行了评估。过氧化氢作为一种点火助燃剂,当少量加入时,可以显著增强爆轰化学反应。它改变了底层爆震波的点火化学性质,而不影响整体热力学性质。本研究的主要目的是评估当过氧化氢以小浓度和大浓度添加到氢-空气混合物中时,气态爆轰的点火促进和\(\text {NO}_{x}\)缓解效果。在目前的工作中,过氧化氢在大量添加(高达10%) is also reported. The results show a visible effect on ignition promotion up to 20,000 ppm. At concentrations higher than 20,000 ppm of \(\text {H}_{2}\text {O}_{2}\), further reduction in the induction length was found to be minimal. The \(\text {NO}_{x}\) emissions were found to decrease for stoichiometric and fuel-lean \(\text {H}_{2}\)-air mixtures, whereas the \(\text {NO}_{x}\) concentration was found to increase for fuel-rich mixtures with the addition of hydrogen peroxide. Thus, the dual behavior exhibited by \(\text {H}_{2}\text {O}_{2}\) is shown to be advantageous as it could potentially mitigate \(\text {NO}_{x}\) emissions at high temperatures for fuel-lean and stoichiometric hydrogen-air mixtures and, at the same time, could sensitize the given mixture for applications in detonation-based combustors.
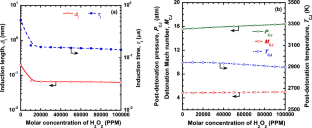
The paper describes the dual behavior observed for hydrogen peroxide when added to hydrogen-air detonating mixtures. The effect of the addition of hydrogen peroxide on \(\text {NO}_{x}\) emissions and critical detonation parameters was evaluated for \(\text {H}_{2}\) air mixtures using one-dimensional ZND calculations. Hydrogen peroxide acts as an ignition promoter and is shown to significantly enhance the detonation chemistry when added in small concentrations. It alters the ignition chemistry of an underlying detonation wave without affecting the bulk thermodynamic properties. The main objective of the present study is to evaluate the ignition promotion and \(\text {NO}_{x}\) mitigation effects of hydrogen peroxide in gaseous detonations when it is added to hydrogen-air mixtures in small and large concentrations. In the current work, the diminishing sensitizing potential of hydrogen peroxide when added in large amounts (up to 10%) is also reported. The results show a visible effect on ignition promotion up to 20,000 ppm. At concentrations higher than 20,000 ppm of \(\text {H}_{2}\text {O}_{2}\), further reduction in the induction length was found to be minimal. The \(\text {NO}_{x}\) emissions were found to decrease for stoichiometric and fuel-lean \(\text {H}_{2}\)-air mixtures, whereas the \(\text {NO}_{x}\) concentration was found to increase for fuel-rich mixtures with the addition of hydrogen peroxide. Thus, the dual behavior exhibited by \(\text {H}_{2}\text {O}_{2}\) is shown to be advantageous as it could potentially mitigate \(\text {NO}_{x}\) emissions at high temperatures for fuel-lean and stoichiometric hydrogen-air mixtures and, at the same time, could sensitize the given mixture for applications in detonation-based combustors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


