用于高能量密度水电池的水合物-熔体电解质
IF 60.1
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 561
摘要
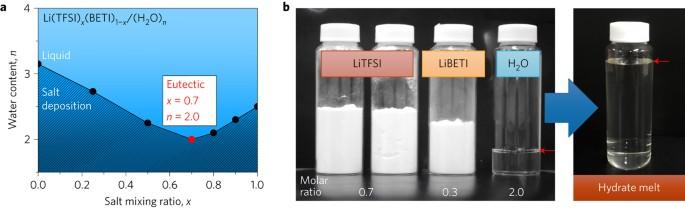
水性锂离子电池因其潜在的低成本、安全和环保特性,正吸引着越来越多的关注。然而,由于水的工作电位窗口较窄,合适的负极选择有限,导致其能量密度较低(根据电极总重量计算为 100 Wh kg-1),这对其未来的广泛应用造成了问题。在此,我们探索了几种有机锂盐的优化共晶体系,并证明锂盐的室温水合物熔体可用作稳定的水性电解质,其中所有水分子都参与 Li+ 水合壳,同时保持流动性。这种水合物熔体电解质可在商用 Li4Ti5O12 负极上进行可逆反应,反应电位低(相对于 Li+/Li 为 1.55 V),容量高(175 mAh g-1)。由此产生的水性锂离子电池具有高能量密度(130 Wh kg-1)和高电压(2.3-3.1 V),在实现与商用非水性电池(能量密度为 150-400 Wh kg-1,电压为 2.4-3.8 V)相媲美的性能方面取得了重大进展。水性锂离子电池的能量密度大大低于非水性电池。作者在此报告了一种室温水合物金属盐电解质,该电解质与尖晶石 Li4Ti5O12 电极结合使用时,能量密度可达 130 Wh kg-1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrate-melt electrolytes for high-energy-density aqueous batteries
Aqueous Li-ion batteries are attracting increasing attention because they are potentially low in cost, safe and environmentally friendly. However, their low energy density (<100 Wh kg−1 based on total electrode weight), which results from the narrow operating potential window of water and the limited selection of suitable negative electrodes, is problematic for their future widespread application. Here, we explore optimized eutectic systems of several organic Li salts and show that a room-temperature hydrate melt of Li salts can be used as a stable aqueous electrolyte in which all water molecules participate in Li+ hydration shells while retaining fluidity. This hydrate-melt electrolyte enables a reversible reaction at a commercial Li4Ti5O12 negative electrode with a low reaction potential (1.55 V versus Li+/Li) and a high capacity (175 mAh g−1). The resultant aqueous Li-ion batteries with high energy density (>130 Wh kg−1) and high voltage (∼2.3–3.1 V) represent significant progress towards performance comparable to that of commercial non-aqueous batteries (with energy densities of ∼150–400 Wh kg−1 and voltages of ∼2.4–3.8 V). Aqueous Li-ion batteries have considerably lower energy density than their non-aqueous counterparts. Here the authors report a room-temperature hydrate metal salt electrolyte that, when coupled with a spinel Li4Ti5O12 electrode, displays an energy density of 130 Wh kg−1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


