磷腈碱t-Bu-P2催化(2,2,2-三氟乙基)芳烃的脱氟转化
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在本研究中,我们证明了1-叔丁基-2,2,4,4,4-五akis(二甲氨基)-2λ5,4 - λ -catenadi(phosphazene) (t- bub - p2)催化(2,2,2-三氟乙基)芳烃与烷腈的去氟官能化反应生成单氟烯烃产品。该反应通过从(2,2,2-三氟乙基)芳烃中消去HF,形成宝石-二氟苯乙烯中间体,随后是亲核加成的烷腈和消去氟阴离子。该催化剂与多种官能团相容。本文章由计算机程序翻译,如有差异,请以英文原文为准。
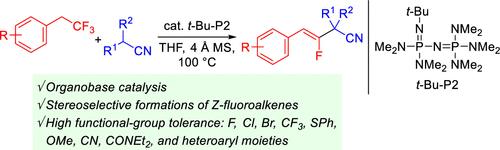
Defluorinative Transformation of (2,2,2-Trifluoroethyl)arenes Catalyzed by the Phosphazene Base t-Bu-P2
In this study, we demonstrated that 1-tert-butyl-2,2,4,4,4-pentakis(dimethylamino)-2λ5,4λ5-catenadi(phosphazene) (t-Bu-P2) catalyzes the defluorinative functionalization reactions of (2,2,2-trifluoroethyl)arenes with alkanenitriles to produce monofluoroalkene products. The reaction proceeds through HF elimination from a (2,2,2-trifluoroethyl)arene to form a gem-difluorostyrene intermediate, which is followed by nucleophilic addition of an alkanenitrile and elimination of a fluoride anion. The catalysis is compatible with a variety of functional groups.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


