双(1-萘基)苊:氧化还原性质和单电子还原
IF 1.5
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
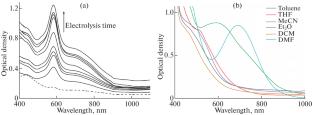
用伏安法研究了双(1-萘基)苊的氧化还原性质。通过电化学还原法制备了二亚胺配体的自由基阴离子,并对其光学性质进行了研究。合成了一种具有双(1-萘基)-苊阴离子自由基形式的新型钠配合物,并对其结构进行了表征。我们发现溶剂性质影响钠配合物的长波吸收带,这是由配位溶剂分子对最低未占据分子轨道(LUMO)能量的贡献决定的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bis(1-naphthylimino)acenaphthene: Redox Properties and One-Electron Reduction
Redox properties of bis(1-naphtylimino)acenaphthene have been studied using various voltammetry techniques. Radical anion of diimine ligand has been obtained in situ through electrochemical reduction, and its optical properties have been studied. A new sodium complex having the anion-radical form of bis(1-naphthylimino)-acenaphthene has been obtained and structurally characterized. We have found that the solvent nature affects the long-wavelength absorption band of the sodium complex, which is determined by the contribution of the coordinated solvent molecules to the lowest unoccupied molecular orbital (LUMO) energy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Physical Chemistry
化学-物理化学
CiteScore
1.50
自引率
0.00%
发文量
9
审稿时长
6-12 weeks
期刊介绍:
Doklady Physical Chemistry is a monthly journal containing English translations of current Russian research in physical chemistry from the Physical Chemistry sections of the Doklady Akademii Nauk (Proceedings of the Russian Academy of Sciences). The journal publishes the most significant new research in physical chemistry being done in Russia, thus ensuring its scientific priority. Doklady Physical Chemistry presents short preliminary accounts of the application of the state-of-the-art physical chemistry ideas and methods to the study of organic and inorganic compounds and macromolecules; polymeric, inorganic and composite materials as well as corresponding processes. The journal is intended for scientists in all fields of chemistry and in interdisciplinary sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


