摘要
严重急性呼吸系统综合征冠状病毒2型感染与急性和急性后神经系统症状有关。新出现的证据表明,严重急性呼吸系统综合征冠状病毒2型可以改变线粒体代谢,这表明大脑代谢的变化可能有助于急性和急性后神经系统并发症的发展。单胺氧化酶B(MAO-B)是一种位于线粒体外膜的黄素酶,对单胺类神经递质的氧化脱氨基进行催化。计算分析显示,ACE2受体上的严重急性呼吸系统综合征冠状病毒2型刺突糖蛋白受体结合结构域与MAO-B之间具有高度相似性,这导致了严重急性呼吸系冠状病毒2型棘突糖蛋白可能通过与MAO-B相互作用来改变神经递质代谢的假设。我们的研究结果根据经验证明,严重急性呼吸系统综合征冠状病毒2型刺突糖蛋白与MAO-B相互作用,导致SH-SY5Y神经元样细胞中MAO-B活性增加。在神经退行性疾病的病理生理机制中,我们还证明了刺突糖蛋白损害线粒体生物能量,诱导氧化应激,并通过线粒体自噬干扰去极化异常线粒体的降解。我们的研究结果还表明,表达严重急性呼吸系统综合征冠状病毒2型刺突蛋白的SH-SY5Y神经元样细胞更容易受到MPTP诱导的坏死,可能是坏死。总之,这些结果揭示了可能导致严重急性呼吸系统综合征冠状病毒2型神经退行性变的新机制。
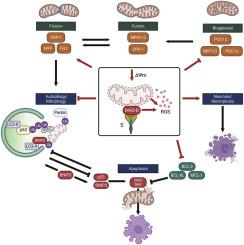
SARS-CoV-2 infection is associated with both acute and post-acute neurological symptoms. Emerging evidence suggests that SARS-CoV-2 can alter mitochondrial metabolism, suggesting that changes in brain metabolism may contribute to the development of acute and post-acute neurological complications. Monoamine oxidase B (MAO-B) is a flavoenzyme located on the outer mitochondrial membrane that catalyzes the oxidative deamination of monoamine neurotransmitters. Computational analyses have revealed high similarity between the SARS-CoV-2 spike glycoprotein receptor binding domain on the ACE2 receptor and MAO-B, leading to the hypothesis that SARS-CoV-2 spike glycoprotein may alter neurotransmitter metabolism by interacting with MAO-B. Our results empirically establish that the SARS-CoV-2 spike glycoprotein interacts with MAO-B, leading to increased MAO-B activity in SH-SY5Y neuron-like cells. Common to neurodegenerative disease pathophysiological mechanisms, we also demonstrate that the spike glycoprotein impairs mitochondrial bioenergetics, induces oxidative stress, and perturbs the degradation of depolarized aberrant mitochondria through mitophagy. Our findings also demonstrate that SH-SY5Y neuron-like cells expressing the SARS-CoV-2 spike protein were more susceptible to MPTP-induced necrosis, likely necroptosis. Together, these results reveal novel mechanisms that may contribute to SARS-CoV-2-induced neurodegeneration.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


