CLDN6特异性CAR-T细胞加扩增RNA疫苗治疗复发或难治性实体瘤:1期BNT211-01试验。
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
癌胚抗原Claudin 6(CLDN6)在许多实体瘤中高度特异性表达,可能是一种有前途的治疗靶点。我们报告了正在进行的1/2期BNT211-01试验的剂量递增结果,该试验评估了在复发/难治性CLDN6阳性实体瘤中,在两个剂量水平(DL)下使用或不使用CAR-T细胞扩增RNA疫苗(CARVac)靶向CLDN6的嵌合抗原受体(CAR)T细胞的安全性和可行性。主要终点是安全性和耐受性、最大耐受剂量和推荐的2期剂量(RP2D)。次要终点包括客观缓解率(ORR)和疾病控制率。我们观察到可控制的毒性,22名患者中有10名(46%)出现细胞因子释放综合征,包括一例3级事件,22名中有1名(5%)出现1级免疫效应细胞相关神经毒性综合征。两名患者在DL较高时出现剂量限制性毒性,无后遗症。CAR-T细胞移植是稳健的,并且CARVac的添加具有良好的耐受性。21名可评估患者中未经证实的ORR为33%(21例中有7例),包括一例完全缓解。疾病控制率为67%(21例中有14例),7例患者病情稳定。以较高DL治疗的生殖细胞肿瘤患者表现出最高的应答率(ORR 57%(4/7))。最大耐受剂量和RP2D尚未确定,因为试验已被修改为使用自动化制造工艺。剂量递增的重复正在进行中,并将确定关键试验的RP2D。ClinicalTrials.gov标识符:NCT04503278。本文章由计算机程序翻译,如有差异,请以英文原文为准。
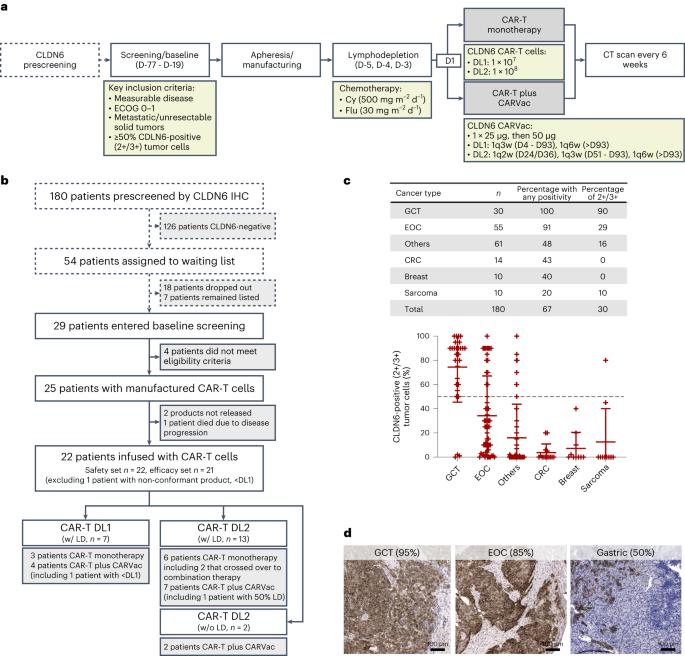
CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial
The oncofetal antigen Claudin 6 (CLDN6) is highly and specifically expressed in many solid tumors, and could be a promising treatment target. We report dose escalation results from the ongoing phase 1/2 BNT211-01 trial evaluating the safety and feasibility of chimeric antigen receptor (CAR) T cells targeting the CLDN6 with or without a CAR-T cell-amplifying RNA vaccine (CARVac) at two dose levels (DLs) in relapsed/refractory CLDN6-positive solid tumors. The primary endpoints were safety and tolerability, maximum tolerated dose and recommended phase 2 dose (RP2D). Secondary endpoints included objective response rate (ORR) and disease control rate. We observed manageable toxicity, with 10 out of 22 patients (46%) experiencing cytokine release syndrome including one grade 3 event and 1 out of 22 (5%) with grade 1 immune effector cell-associated neurotoxicity syndrome. Dose-limiting toxicities occurred in two patients at the higher DL, resolving without sequelae. CAR-T cell engraftment was robust, and the addition of CARVac was well tolerated. The unconfirmed ORR in 21 evaluable patients was 33% (7 of 21), including one complete response. The disease control rate was 67% (14 of 21), with stable disease in seven patients. Patients with germ cell tumors treated at the higher DL exhibited the highest response rate (ORR 57% (4 of 7)). The maximum tolerated dose and RP2D were not established as the trial has been amended to utilize an automated manufacturing process. A repeat of the dose escalation is ongoing and will identify a RP2D for pivotal trials. ClinicalTrials.gov Identifier: NCT04503278 . In the ongoing phase 1/2 BNT211-01 trial, CLDN6-specific chimeric antigen receptor (CAR)-T cells given with or without CARVac, a CAR-T cell-amplifying RNA vaccine, were well-tolerated and exhibited encouraging clinical activity in patients with relapsed or refractory CLDN6-positive solid tumors, with the highest response rate in patients with germ cell tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


