PKNOX1作为DHH的转录因子,通过调节Hedgehog信号通路促进胃腺癌的进展。
IF 3.5
3区 医学
International Journal of Immunopathology and Pharmacology
Pub Date : 2023-01-01
DOI:10.1177/03946320231208833
引用次数: 0
摘要
背景:本研究探讨了PBX/打结1同源盒1(PKNOX1)可能加重胃腺癌(STAD)的作用和潜在机制。方法:为了进行计算机分析,我们使用UALCAN网站检测了TCGA-PKNOX1的表达,以及它在从GEO数据库获得的GSE172032和GSE174237数据集中的表达模式。通过KMplot网络工具分析相关的患者生存曲线。在体外,我们分别使用细胞计数试剂盒-8、集落形成、伤口愈合和细胞迁移测定来测量细胞活力、增殖、迁移和侵袭。实时qPCR和蛋白质印迹评估了PKNOX1、蜗牛、波形蛋白、N-钙粘蛋白、E-钙粘素、沙漠刺猬(DHH)、细胞周期蛋白D2、神经胶质瘤相关癌基因同源物1的mRNA和蛋白质水平,并使其平滑。使用LinkedOmics网络工具和R中的clusterProfiler软件包进行基因集富集分析。双荧光素酶报告基因分析用于检测PKNOX1与DHH的相互作用,以及TEA结构域转录因子4(TEAD4)与PKNOX1的相互作用。结果:PKNOX1在STAD中高表达,与患者生存率低有关。PKNOX1的下调抑制了STAD细胞的活力、增殖、迁移、侵袭和上皮-间质转化。TEAD4的上调促进了菌落的形成和迁移,而PKNOX1的耗竭则逆转了这些作用。此外,PKNOX1在基因水平上调节刺猬信号通路的激活,因为我们确定PKNOX1是促进其表达的DHH的假定转录因子。结论:我们的研究结果表明,PKNOX1是DHH的候选转录因子,并通过调节刺猬信号通路促进STAD的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PKNOX1 acts as a transcription factor of DHH and promotes the progression of stomach adenocarcinoma by regulating the Hedgehog signalling pathway.
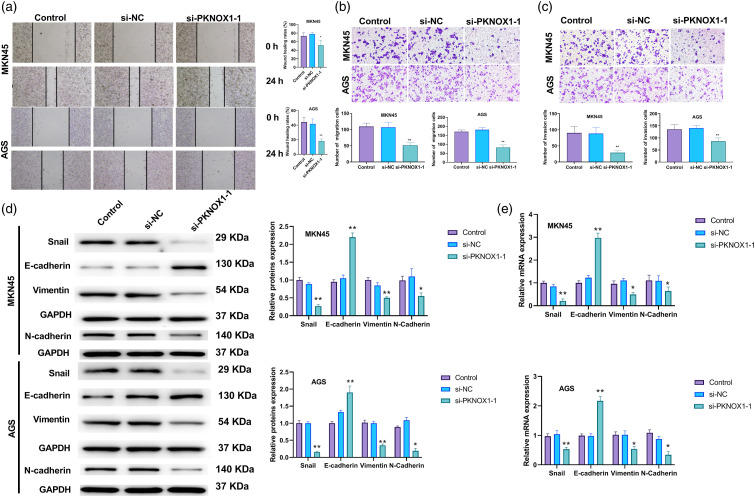
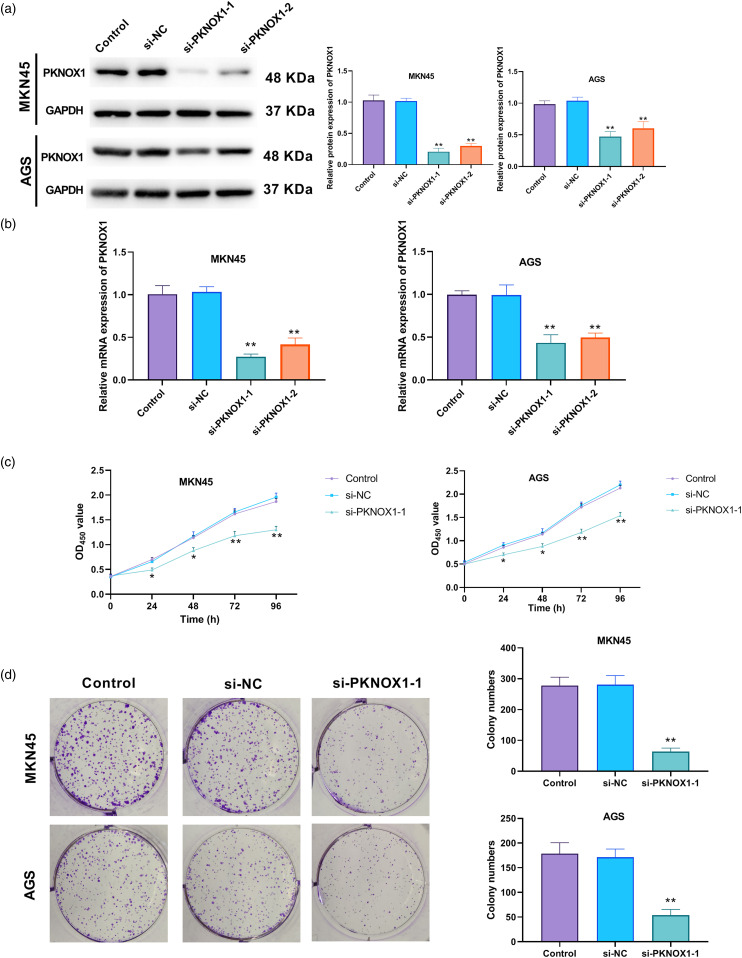
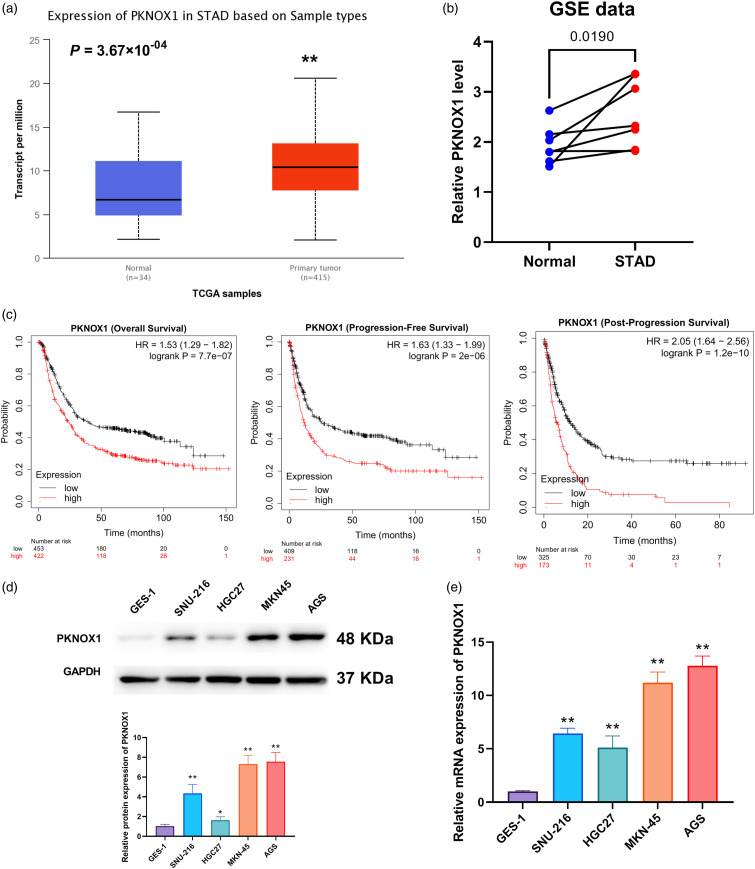
Background: This study explored the effects and potential mechanism by which PBX/knotted 1 homeobox 1 (PKNOX1) may exacerbate stomach adenocarcinoma (STAD). Methods: For the in silico analysis, we examined TCGA-PKNOX1 expression using the UALCAN website, as well as its expression patterns in the GSE172032 and GSE174237 datasets, obtained from the GEO database. The associated patient survival curves, were analysed via the KMplot webtool. In vitro, we measured cell viability, proliferation, migration, and invasion using cell counting kit-8, colony formation, wound healing, and cell migration assays, respectively. Real time qPCR and western blotting assessed the mRNA and protein levels of PKNOX1, Snail, vimentin, N-cadherin, E-cadherin, desert hedgehog (DHH), cyclin D2, glioma-associated oncogene homolog 1, and smoothened. Gene Set Enrichment Analysis was performed using LinkedOmics webtools and the clusterProfiler package in R. Dual-luciferase reporter assay was used to examine the interactions of PKNOX1 with DHH, and of TEA domain transcription factor 4 (TEAD4) with PKNOX1. Results: PKNOX1 was highly expressed in STAD and linked to poor patient survival. Downregulation of PKNOX1 inhibited STAD cell viability, proliferation, migration, invasion, and epithelial-mesenchymal transition. Upregulation of TEAD4 promoted colony formation and migration, while these effects were reversed by PKNOX1 depletion. Furthermore, PKNOX1 regulated the activation of the hedgehog signalling pathway at the gene level, as we identified PKNOX1 to be a putative transcription factor for DHH that promotes its expression. Conclusion: Our results show that PKNOX1 acts as a candidate transcription factor for DHH and facilitates STAD development by regulating the hedgehog signalling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Immunopathology and Pharmacology
Immunology and Microbiology-Immunology
自引率
0.00%
发文量
88
期刊介绍:
International Journal of Immunopathology and Pharmacology is an Open Access peer-reviewed journal publishing original papers describing research in the fields of immunology, pathology and pharmacology. The intention is that the journal should reflect both the experimental and clinical aspects of immunology as well as advances in the understanding of the pathology and pharmacology of the immune system.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


