骨髓小生境的细胞可塑性促进造血干细胞再生。
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
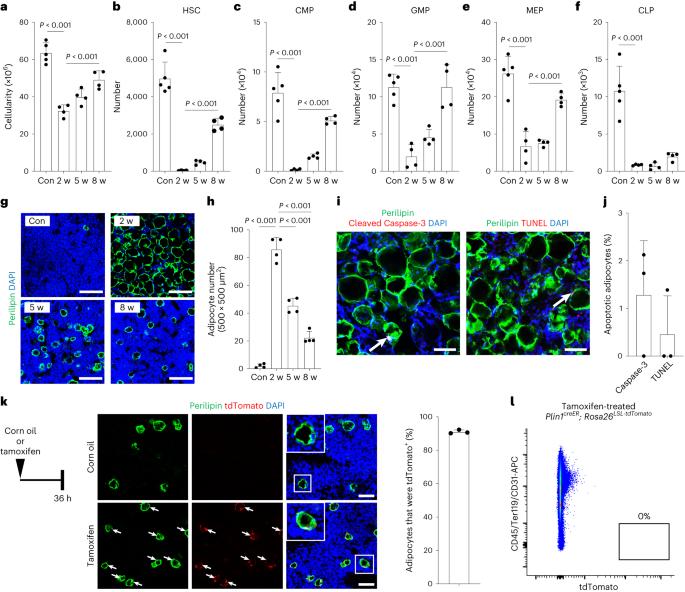
骨髓消融术后造血干细胞(HSC)再生,这一过程会对骨髓产生不利影响,并促使瘦素受体表达细胞(一个关键的小生境成分)广泛分化为脂肪细胞。骨髓小生境的再生与脂肪细胞的分解有关,但其机制尚不清楚。在小鼠中使用Plin1-creER敲除,我们跟踪了体内再生生态位中脂肪细胞的命运。我们发现骨髓脂肪细胞在骨髓消融术后的再生过程中是高度动态的,并去分化为瘦素受体表达细胞。骨骼损伤后,骨髓脂肪细胞可产生骨谱系细胞。稳态骨髓脂肪细胞的细胞命运也是可塑的。骨髓基质细胞(包括脂肪细胞)中脂肪甘油三酯脂肪酶(Atgl)的缺失阻碍了脂肪细胞的去分化,并导致造血干细胞的再生严重受损,骨髓消融术后B淋巴细胞生成受损,但不处于稳定状态。因此,HSC及其生态位的再生取决于骨髓脂肪细胞的细胞可塑性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cellular plasticity of the bone marrow niche promotes hematopoietic stem cell regeneration
Hematopoietic stem cells (HSCs) regenerate after myeloablation, a procedure that adversely disrupts the bone marrow and drives leptin receptor-expressing cells, a key niche component, to differentiate extensively into adipocytes. Regeneration of the bone marrow niche is associated with the resolution of adipocytes, but the mechanisms remain poorly understood. Using Plin1-creER knock-in mice, we followed the fate of adipocytes in the regenerating niche in vivo. We found that bone marrow adipocytes were highly dynamic and dedifferentiated to leptin receptor-expressing cells during regeneration after myeloablation. Bone marrow adipocytes could give rise to osteolineage cells after skeletal injury. The cellular fate of steady-state bone marrow adipocytes was also plastic. Deletion of adipose triglyceride lipase (Atgl) from bone marrow stromal cells, including adipocytes, obstructed adipocyte dedifferentiation and led to severely compromised regeneration of HSCs as well as impaired B lymphopoiesis after myeloablation, but not in the steady state. Thus, the regeneration of HSCs and their niche depends on the cellular plasticity of bone marrow adipocytes. Mouse lineage tracing in regenerating bone marrow after myeloablation shows a dynamic dedifferentiation of mature adipocytes into bone marrow stromal cells. Lipolysis disruption obstructs adipocyte dedifferentiation and hematopoietic stem cell regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


