下载PDF
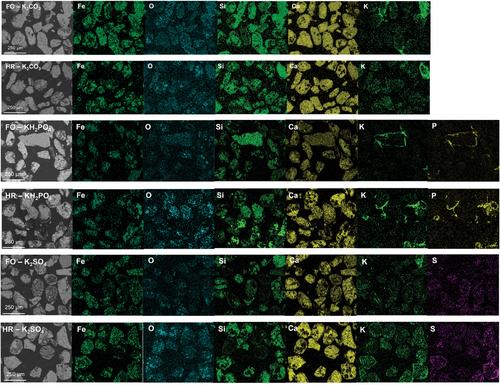
{"title":"基于不同初始氧化态的天然和废料的钾灰与氧载体之间的相互作用","authors":"Victor Purnomo, Fredrik Hildor, Pavleta Knutsson, Henrik Leion","doi":"10.1002/ghg.2208","DOIUrl":null,"url":null,"abstract":"<p>One of the most essential features of an oxygen carrier is its ability to be oxidized and reduced in order to transfer oxygen in a chemical looping system. A highly reduced oxygen carrier can experience multiple performance issues, such as decreased reactivity, agglomeration, and defluidization. This is crucial for processes that require limited oxygen transfer from the air reactor to the fuel reactor. Meanwhile, biomasses as environmentally friendly fuel options contain ashes, which would inevitably react with oxygen carriers and exacerbate the performance issues. To mimic the interactions between a highly reduced oxygen carrier and biomass ash compounds, four iron-based oxygen carriers, based on natural ores and waste materials, and three potassium salts, K<sub>2</sub>CO<sub>3</sub>, KH<sub>2</sub>PO<sub>4</sub>, and K<sub>2</sub>SO<sub>4</sub>, were investigated in a tubular reactor under an atmosphere consisting of 2.5% H<sub>2</sub> and 10% steam in Ar and N<sub>2</sub> at 900°C for 3 h. The results from the X-ray diffraction (XRD) material analysis showed that both initially fully oxidized and highly reduced materials reach the same oxidation state after the experiment. Based on the scanning electron microscopy coupled with energy dispersive X-ray spectroscopy results, K from K<sub>2</sub>CO<sub>3</sub> and K<sub>2</sub>SO<sub>4</sub> diffuses in the oxygen carrier particles, while K from KH<sub>2</sub>PO<sub>4</sub> always forms a distinct layer around the particles. The initial oxidation state of an oxygen carrier surface affects the interactions with the potassium salt only to minor extents. Thus, the final state of the material and its performance in a large-scale process are only occasionally and mildly affected by its initial oxidation state. © 2023 The Authors. <i>Greenhouse Gases: Science and Technology</i> published by Society of Chemical Industry and John Wiley & Sons Ltd.</p>","PeriodicalId":12796,"journal":{"name":"Greenhouse Gases: Science and Technology","volume":"13 4","pages":"520-534"},"PeriodicalIF":2.7000,"publicationDate":"2023-03-11","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ghg.2208","citationCount":"1","resultStr":"{\"title\":\"Interactions between potassium ashes and oxygen carriers based on natural and waste materials at different initial oxidation states\",\"authors\":\"Victor Purnomo, Fredrik Hildor, Pavleta Knutsson, Henrik Leion\",\"doi\":\"10.1002/ghg.2208\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>One of the most essential features of an oxygen carrier is its ability to be oxidized and reduced in order to transfer oxygen in a chemical looping system. A highly reduced oxygen carrier can experience multiple performance issues, such as decreased reactivity, agglomeration, and defluidization. This is crucial for processes that require limited oxygen transfer from the air reactor to the fuel reactor. Meanwhile, biomasses as environmentally friendly fuel options contain ashes, which would inevitably react with oxygen carriers and exacerbate the performance issues. To mimic the interactions between a highly reduced oxygen carrier and biomass ash compounds, four iron-based oxygen carriers, based on natural ores and waste materials, and three potassium salts, K<sub>2</sub>CO<sub>3</sub>, KH<sub>2</sub>PO<sub>4</sub>, and K<sub>2</sub>SO<sub>4</sub>, were investigated in a tubular reactor under an atmosphere consisting of 2.5% H<sub>2</sub> and 10% steam in Ar and N<sub>2</sub> at 900°C for 3 h. The results from the X-ray diffraction (XRD) material analysis showed that both initially fully oxidized and highly reduced materials reach the same oxidation state after the experiment. Based on the scanning electron microscopy coupled with energy dispersive X-ray spectroscopy results, K from K<sub>2</sub>CO<sub>3</sub> and K<sub>2</sub>SO<sub>4</sub> diffuses in the oxygen carrier particles, while K from KH<sub>2</sub>PO<sub>4</sub> always forms a distinct layer around the particles. The initial oxidation state of an oxygen carrier surface affects the interactions with the potassium salt only to minor extents. Thus, the final state of the material and its performance in a large-scale process are only occasionally and mildly affected by its initial oxidation state. © 2023 The Authors. <i>Greenhouse Gases: Science and Technology</i> published by Society of Chemical Industry and John Wiley & Sons Ltd.</p>\",\"PeriodicalId\":12796,\"journal\":{\"name\":\"Greenhouse Gases: Science and Technology\",\"volume\":\"13 4\",\"pages\":\"520-534\"},\"PeriodicalIF\":2.7000,\"publicationDate\":\"2023-03-11\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ghg.2208\",\"citationCount\":\"1\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Greenhouse Gases: Science and Technology\",\"FirstCategoryId\":\"93\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/ghg.2208\",\"RegionNum\":4,\"RegionCategory\":\"环境科学与生态学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q3\",\"JCRName\":\"ENERGY & FUELS\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Greenhouse Gases: Science and Technology","FirstCategoryId":"93","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/ghg.2208","RegionNum":4,"RegionCategory":"环境科学与生态学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"ENERGY & FUELS","Score":null,"Total":0}
引用次数: 1
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


