无环葫芦烷及其应用
IF 2.3
4区 化学
Q2 Agricultural and Biological Sciences
Journal of Inclusion Phenomena and Macrocyclic Chemistry
Pub Date : 2022-08-23
DOI:10.1007/s10847-022-01159-w
引用次数: 1
摘要
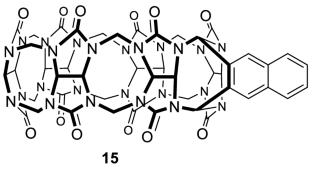
无环葫芦[n]urils是一种新型的葫芦类衍生物,由一个中心糖脲四聚体骨架、两个取代的末端芳香环和四个带增溶基团的连接体组成。由于其灵活的构象和易于功能化,无环葫芦[n]环对各种客体具有高的结合亲和力和选择性。本文综述了无环葫芦[n]环的结构与性质之间的关系。近年来无环葫芦[n]urils的发展为制药和生化领域的实际应用铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acyclic cucurbiturils and their applications
Acyclic cucurbit[n]urils are a novel type of cucurbituril derivatives, consisting of a central glycoluril tetramer skeleton, two substituted terminal aromatic rings, and four linkers bearing solubilizing groups. Due to flexible conformation and facile functionalization, acyclic cucurbit[n]urils are tailored to exhibit high binding affinity and selectivity toward a variety of guests. In this review, we focus on the relationship between structure and property of acyclic cucurbit[n]urils. The recent development of acyclic cucurbit[n]urils has paved the way for practical applications in pharmaceutical and biochemical areas.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.30
自引率
8.70%
发文量
0
审稿时长
3-8 weeks
期刊介绍:
The Journal of Inclusion Phenomena and Macrocyclic Chemistry is the premier interdisciplinary publication reporting on original research into all aspects of host-guest systems. Examples of specific areas of interest are: the preparation and characterization of new hosts and new host-guest systems, especially those involving macrocyclic ligands; crystallographic, spectroscopic, thermodynamic and theoretical studies; applications in chromatography and inclusion polymerization; enzyme modelling; molecular recognition and catalysis by inclusion compounds; intercalates in biological and non-biological systems, cyclodextrin complexes and their applications in the agriculture, flavoring, food and pharmaceutical industries; synthesis, characterization and applications of zeolites.
The journal publishes primarily reports of original research and preliminary communications, provided the latter represent a significant advance in the understanding of inclusion science. Critical reviews dealing with recent advances in the field are a periodic feature of the journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


