从苍术的根状茎中分离出三种具有前所未有的C - C连锁的苦参甾醇倍半萜酚类化合物——苍术类A−C
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 1
摘要
从苍术的根状茎中分离得到3个倍半萜酚类化合物,atramacronoids A−C(1−3),具有罕见的6/6/5/5/6骨架,形成了一个意想不到的C-8−C-16连锁。通过光谱数据分析、化学计算和x射线衍射分析,确定了它们的结构和绝对构型。提出了化合物1 ~ 3的可行生物合成途径。令人惊讶的是,化合物1通过诱导细胞凋亡对SGC-7901细胞表现出细胞毒性,这可能与促进中性粒细胞弹性酶的合成有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
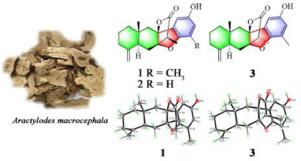
Atramacronoids A−C, three eudesmanolide sesquiterpene-phenol hybrids with an unprecedented C−C linkage from the rhizomes of Atractylodes macrocephala
Three eudesmanolide sesquiterpene-phenol hybrids, atramacronoids A−C (1−3), featuring an unusual 6/6/5/5/6 skeleton furnished by forming an unexpected C-8−C-16 linkage, were obtained from the rhizomes of Atractylodes macrocephala. Their structures and absolute configurations were elucidated by spectroscopic data analysis, chemical calculations, combined with X-ray diffractions. The plausible biosynthetic pathways for compounds 1−3 are proposed. Surprisingly, compound 1 exhibited cytotoxicity against SGC-7901 cells by inducing cells apoptosis, which might relate to the promotion of synthesis of neutrophil elastase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


