醋酸锌对可持续水电池电解质的亲水增溶作用
IF 27.1
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
在可持续性更强的电池化学材料中,锌水溶液系统再次受到关注。为了加快这项前景广阔的技术的实际应用,一种有效的策略是采用高浓度盐电解质,以解决关键的技术障碍,特别是阳极侧的氢进化反应和枝晶生长。然而,最先进的配方要么缺乏锌离子,要么依赖卤素盐,不幸的是,这两种情况都带来了额外的挑战。在这里,我们展示了一种利用醋酸锌的高浓度水性电解液配方,醋酸锌是一种水溶性很差但却廉价环保的盐。前所未有的溶解度(高达 23 米)是由于引入了亲水剂,将醋酸阴离子配体转变为亲水配位结构。所有三种亲水剂(包括醋酸钾、尿素和乙酰胺)都能有效地构建高浓度醋酸锌电解质,使用这些电解质组装的锌//芘-4,5,9,10-四酮全电池在循环 4,000 次后仍能保持 70% 的初始容量。这项研究为设计高性能电解质应用于宽电池领域提供了一个独特的机会。作者采用了一种水溶解策略,将原本水溶性很差的醋酸盐转化为高浓度水性电解质的盐,这种电解质具有成本低、环境可持续性强的特点,并能使锌电池发挥良好的性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
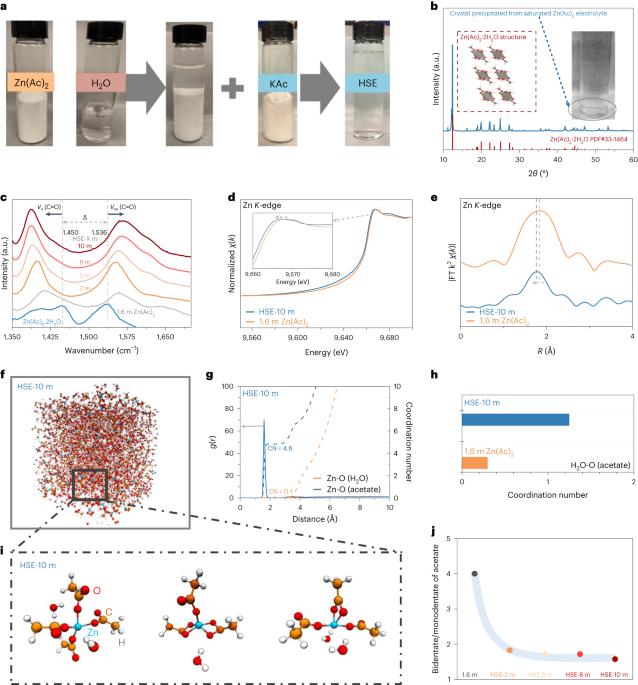
Hydrotropic solubilization of zinc acetates for sustainable aqueous battery electrolytes
Among the more sustainable battery chemistries, the aqueous zinc system is receiving renewed interest. To accelerate the practical applications of this promising technology, an effective strategy is to deploy high salt concentration electrolytes that could address the critical technical barriers, notably hydrogen evolution reaction and dendrite growth at the anode side. However, the state-of-the-art recipes are either zinc-ion deficient or halogen salt dependent, both of which unfortunately create extra challenges. Here we show a highly concentrated aqueous electrolyte formula utilizing zinc acetate, an otherwise poorly water-soluble but cheap and eco-friendly salt. The unprecedented solubility (up to 23 m) is a result of the introduction of hydrotropic agents that transform the acetate anion ligands to a hydrophilic coordination structure. All three hydrotropic agents including potassium acetate, urea and acetamide are effective in constructing highly concentrated zinc acetate electrolytes with which the assembled Zn//pyrene-4,5,9,10-tetraone full cell retains 70% of its initial capacity after 4,000 cycles. This work provides a unique opportunity to design high-performance electrolytes for applications in the wide battery space. The authors deploy a hydrotropic solubilization strategy turning an otherwise poorly water-soluble acetate into a salt for a high-concentration aqueous electrolyte that features low cost and environmental sustainability and enables good performance of zinc batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Sustainability
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
41.90
自引率
1.10%
发文量
159
期刊介绍:
Nature Sustainability aims to facilitate cross-disciplinary dialogues and bring together research fields that contribute to understanding how we organize our lives in a finite world and the impacts of our actions.
Nature Sustainability will not only publish fundamental research but also significant investigations into policies and solutions for ensuring human well-being now and in the future.Its ultimate goal is to address the greatest challenges of our time.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


