原位自组装用于癌症治疗和成像
IF 79.8
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
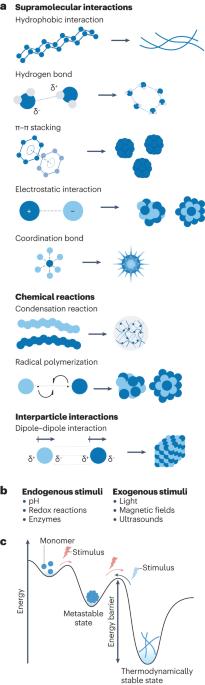
原位自组装--通过单体的生化反应在原位形成复杂材料--提高了癌症治疗和成像的给药效果。迄今为止,纳米医学一直局限于原位自组装,这是因为原位自组装的肿瘤深层渗透性差,血液循环不畅。相比之下,基于原位自组装的癌症治疗方法具有各种优势,包括增强单体的血液循环、长期给药的药代动力学、低耐药性以及靶向深部肿瘤和细胞器的能力,这可以导致破坏介导的细胞凋亡,并使细胞活动成像成为可能,从而实现有效的癌症治疗和诊断。在本综述中,我们将讨论基于细胞外和细胞内环境中的各种内源性和外源性刺激,设计用于癌症治疗和成像的原位自组装纳米药物。我们还强调了通过原位自组装纳米结构的多模式动态转化进行癌症治疗的优势,包括诱导机械应力、部署癌症特异性靶向疗法、获得深层肿瘤渗透以及延长药物在体内的保留时间。最后,我们从临床角度讨论了原位自组装纳米药物所面临的挑战及其为现有癌症疗法提供先进替代品的潜力。原位自组装在癌症治疗和成像方面具有优势,因为所产生的纳米药物具有高效的深层肿瘤靶向性、增强的血液循环和可忽略的耐药性。本综述将讨论基于内源性和外源性刺激的细胞外和细胞内原位自组装在癌症治疗和成像方面的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

In situ self-assembly for cancer therapy and imaging
In situ self-assembly — the in situ formation of complex materials via biochemical reactions of monomers — has enhanced the efficacy of drug delivery for cancer therapy and imaging. So far, nanomedicine has been confined to ex situ self-assembly, which is limited by poor deep-tumour penetration and poor blood circulation. By contrast, in situ self-assembly-based cancer treatments offer various advantages, including enhanced blood circulation of monomers, long-term drug delivery pharmacokinetics, low drug resistance and the ability to target deep tumours and organelles, which can result in disruption-mediated apoptosis and enable the imaging of cellular activity for effective cancer therapy and diagnosis. In this Review, we discuss the design of in situ self-assembled nanomedicines for cancer therapy and imaging based on various endogenous and exogenous stimuli in both the extracellular and the intracellular milieu. We also highlight the advantages of cancer treatment via multimodal dynamic transformations of nanostructures self-assembled in situ, including the ability to induce mechanical stress, deploy cancer-specific targeted therapies, obtain deep-tumour penetration and sustain prolonged drug retention time in the body. Finally, we discuss from a clinical viewpoint the challenges of in situ self-assembled nanomedicine and its potential to offer advanced alternatives to existing cancer therapies. In situ self-assembly is advantageous for cancer therapy and imaging because of the efficient deep-tumour targeting, enhanced blood circulation and negligible drug resistance of the resulting nanomedicines. This Review discusses extracellular and intracellular in situ self-assembly based on endogenous and exogenous stimuli for cancer therapy and imaging applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Materials
Materials Science-Biomaterials
CiteScore
119.40
自引率
0.40%
发文量
107
期刊介绍:
Nature Reviews Materials is an online-only journal that is published weekly. It covers a wide range of scientific disciplines within materials science. The journal includes Reviews, Perspectives, and Comments.
Nature Reviews Materials focuses on various aspects of materials science, including the making, measuring, modelling, and manufacturing of materials. It examines the entire process of materials science, from laboratory discovery to the development of functional devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


