反相高效液相色谱法研究莫那匹韦胶囊体外释放度
IF 1.5
4区 医学
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
2019冠状病毒病(COVID-19)在全球爆发,促使研究人员努力开发治疗和预防措施,以控制其进展。Molnupiravir是合成核苷衍生物n -4-羟基胞苷的前药,被发现是抗严重急性呼吸综合征冠状病毒2 (SARS-CoV-2)的有希望的候选药物。结果:该方法可显著降低SARS-CoV-2阳性报告患者的住院和死亡风险。本研究建立了紫外检测的反相高效液相色谱法测定其在胶囊剂型中的溶出度和释放度。开发的方法按照国际协调理事会(ICH)指南进行了验证。采用不同的参数对该方法的适用性进行了评价和验证。该方法简便、快速、选择性好、灵敏、准确、精密度高、稳健性好、坚固耐用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
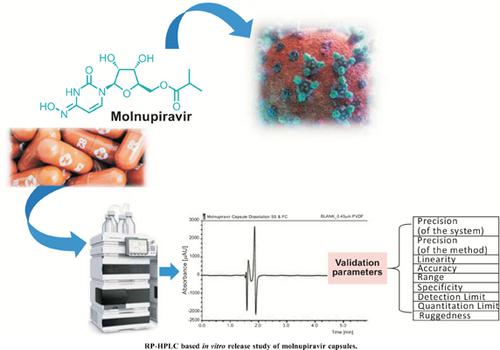
Development and Validation of In-vitro Release Study of Molnupiravir Capsules by RP-HPLC
The coronavirus disease-2019 (COVID-19) outbreak all over the world has led researchers to strive to develop treatment and preventive measures to control its progression.
Molnupiravir, a prodrug of the synthetic nucleoside derivative N-4-hydroxycytidine was found to be a promising candidate against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
Result: It could significantly reduce the risk of hospitalization and mortality among patients with positive SARS-CoV-2 reports. In this study, an RP-HPLC method with UV detection was developed to determine its dissolution and release in the capsule dosage form. The developed method was validated as per International Council for Harmonization (ICH) guidelines.
The method was evaluated and validated for its applicability using various parameters. It was found to be a simple, rapid, selective, sensitive, accurate, precise, robust and rugged method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.50
自引率
0.00%
发文量
85
审稿时长
3 months
期刊介绍:
Aims & Scope
Current Pharmaceutical Analysis publishes expert reviews and original research articles on all the most recent advances in pharmaceutical and biomedical analysis. All aspects of the field are represented including drug analysis, analytical methodology and instrumentation. The journal is essential to all involved in pharmaceutical, biochemical and clinical analysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


