机器学习与多模态神经成像数据分类阿尔茨海默病的阶段:系统回顾和荟萃分析
摘要
近年来,阿尔茨海默病(AD)已严重威胁人类健康。研究人员和临床医生在试图准确识别和划分阿尔茨海默病的阶段时都遇到了巨大的障碍。多项研究表明,多模态神经成像输入可以帮助人们深入了解与阿兹海默症有关的大脑结构和功能变化。机器学习(ML)算法可以利用强大的计算方法识别多模态神经影像数据中的模式和联系,从而准确地对 AD 阶段进行分类。本研究旨在评估 ML 方法对使用多模态神经影像数据准确划分 AD 阶段的贡献。我们在 IEEE Xplore、Science Direct/Elsevier、ACM DigitalLibrary 和 PubMed 数据库中进行了系统搜索,并在 Google Scholar 上进行了前向滚雪球式搜索。定量分析使用了 47 项研究。对所选研究中使用的分类算法和融合方法进行了可解释性分析。通过对多模态神经影像数据和 ML 方法在 AD 分期分类中的分层汇总接收器操作特征(ROC)曲线进行基于双变量模型的荟萃分析,评估了汇总的敏感性和特异性,包括诊断效率。Wilcoxon 符号秩检验进一步用于统计比较现有模型的准确性得分。在 95% 置信区间(78.87%-87.71%)内,将轻度认知障碍(MCI)患者与健康对照(NC)患者区分开来的综合灵敏度为 83.77%;将 AD 患者与 NC 患者区分开来的综合灵敏度为 94.60% (90.76%, 96.89%);将进行性 MCI(pMCI)患者与稳定型 MCI(sMCI)患者区分开来的综合灵敏度为 80.41% (74.73%, 85.06%)。在 95% 的置信区间(78.87%,87.71%)内,区分轻度认知障碍(MCI)和健康对照(NC)参与者的汇总灵敏度为 83.77%,在 95% 的置信区间(90.76%,96.89%)内,区分轻度认知障碍(MCI)和健康对照(NC)参与者的汇总灵敏度为 83.77%。将 AD 与 NC 区分开来的汇总灵敏度为 94.60%,将 MCI 与健康对照(NC)参与者区分开来的汇总灵敏度为 83.77%,将进行性 MCI(pMCI)与稳定型 MCI(sMCI)区分开来的汇总灵敏度为 80.41%(74.73%,85.06%),将早期 MCI(EMCI)与 NC 区分开来的汇总灵敏度为 86.63%(82.43%,89.95%)。区分 MCI 和 NC 的汇总特异性为 79.16% (70.97%, 87.71%),区分 AD 和 NC 的汇总特异性为 93.49% (91.60%, 94.90%),区分 pMCI 和 sMCI 的汇总特异性为 81.44% (76.32%, 85.66%),区分 EMCI 和 NC 的汇总特异性为 85.68% (81.62%, 88.96%)。Wilcoxon 符号秩检验显示,所有分类任务的 P 值都很低。多模态神经影像数据与 ML 在对 AD 分期进行分类方面前景广阔,但要提高其在临床实践中应用的有效性,还需要更多的研究。
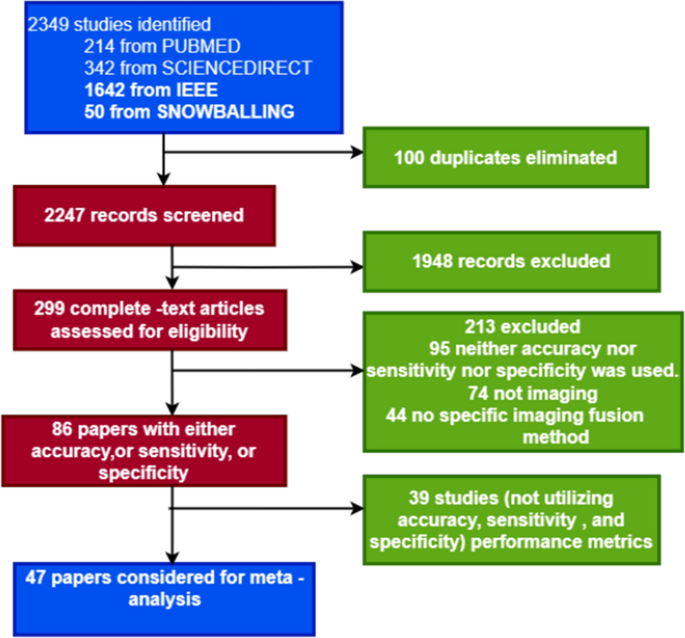
In recent years, Alzheimer's disease (AD) has been a serious threat to human health. Researchers and clinicians alike encounter a significant obstacle when trying to accurately identify and classify AD stages. Several studies have shown that multimodal neuroimaging input can assist in providing valuable insights into the structural and functional changes in the brain related to AD. Machine learning (ML) algorithms can accurately categorize AD phases by identifying patterns and linkages in multimodal neuroimaging data using powerful computational methods. This study aims to assess the contribution of ML methods to the accurate classification of the stages of AD using multimodal neuroimaging data. A systematic search is carried out in IEEE Xplore, Science Direct/Elsevier, ACM DigitalLibrary, and PubMed databases with forward snowballing performed on Google Scholar. The quantitative analysis used 47 studies. The explainable analysis was performed on the classification algorithm and fusion methods used in the selected studies. The pooled sensitivity and specificity, including diagnostic efficiency, were evaluated by conducting a meta-analysis based on a bivariate model with the hierarchical summary receiver operating characteristics (ROC) curve of multimodal neuroimaging data and ML methods in the classification of AD stages. Wilcoxon signed-rank test is further used to statistically compare the accuracy scores of the existing models. With a 95% confidence interval of 78.87-87.71%, the combined sensitivity for separating participants with mild cognitive impairment (MCI) from healthy control (NC) participants was 83.77%; for separating participants with AD from NC, it was 94.60% (90.76%, 96.89%); for separating participants with progressive MCI (pMCI) from stable MCI (sMCI), it was 80.41% (74.73%, 85.06%). With a 95% confidence interval (78.87%, 87.71%), the Pooled sensitivity for distinguishing mild cognitive impairment (MCI) from healthy control (NC) participants was 83.77%, with a 95% confidence interval (90.76%, 96.89%), the Pooled sensitivity for distinguishing AD from NC was 94.60%, likewise (MCI) from healthy control (NC) participants was 83.77% progressive MCI (pMCI) from stable MCI (sMCI) was 80.41% (74.73%, 85.06%), and early MCI (EMCI) from NC was 86.63% (82.43%, 89.95%). Pooled specificity for differentiating MCI from NC was 79.16% (70.97%, 87.71%), AD from NC was 93.49% (91.60%, 94.90%), pMCI from sMCI was 81.44% (76.32%, 85.66%), and EMCI from NC was 85.68% (81.62%, 88.96%). The Wilcoxon signed rank test showed a low P-value across all the classification tasks. Multimodal neuroimaging data with ML is a promising future in classifying the stages of AD but more research is required to increase the validity of its application in clinical practice.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


