MFe2O4铁氧体(M(II) = Fe, Mg, Mn, Zn)的酸碱性质对乙醇转化为丙酮选择性的影响
IF 0.7
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
采用程序升温解吸NH3和CO2对尖晶石结构的MFe2O4 (M(II) = Fe, Mg, Mn, Zn)铁氧体进行了酸碱度表征。结果表明,中等强度的碱性位点和酸性位点在铁氧体上乙醇制丙酮的过程中都起着重要作用。乙醇转化为丙酮的选择性既取决于表面的酸碱性质,也取决于铁氧体表面氧参与丙酮形成和蒸汽重整中间氧化还原阶段的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
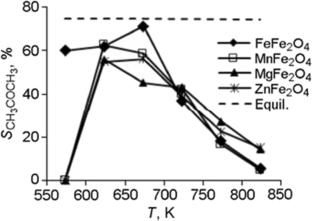
Influence of Acid–Base Properties of MFe2O4 Ferrites (M(II) = Fe, Mg, Mn, Zn) on Their Selectivity in the Conversion of Ethanol to Acetone
The acidity and basicity of MFe2O4 (M(II) = Fe, Mg, Mn, Zn) ferrites of the spinel structure have been characterized by temperature-programmed desorption of NH3 and CO2. It is shown that both medium-strength basic and acid sites play an important role in the process of acetone obtaining from ethanol over ferrites. The selectivity of ethanol conversion to acetone depends both on the acid–base properties of the surface and on the ability of the surface oxygen of ferrite to participate in the intermediate redox stages of formation and steam reforming of acetone.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


