半导体氢传感器用纳米Pd/SnO2材料
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
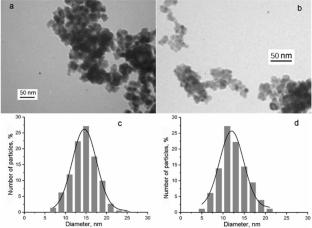
为了制造高灵敏度的半导体氢传感器,采用溶胶-凝胶法制备了粒径为5-6 nm和10-11 nm的纳米二氧化锡,并在其基础上掺杂了pd材料。以含0.24%钯的纳米前草酸盐SnO2为材料制成的传感器对氢浓度的灵敏度最高。所获得的半导体传感器的高灵敏度是由表面工艺对其电阻的显著影响所解释的,因为SnO2颗粒的尺寸最小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nanosized Pd/SnO2 Materials for Semiconductor Hydrogen Sensors
To create highly sensitive semiconductor hydrogen sensors, nanosized tin dioxides with particle sizes of 5-6 and 10-11 nm have been obtained by the sol-gel method using various precursors, as well as Pd-doped materials on their base. The maximum sensitivity to the hydrogen microconcentrations has been found for the sensors based on the nanosized ex-oxalate SnO2 containing 0.24% palladium. High sensitivity of the obtained semiconductor sensors is explained by the significant influence of surface processes on their electrical resistance due to the smallest sizes of the SnO2 particles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


