阴离子存在下牛血清白蛋白(BSA)分子间的相互作用:小角中子散射研究
IF 2.2
4区 生物学
Q3 BIOPHYSICS
引用次数: 1
摘要
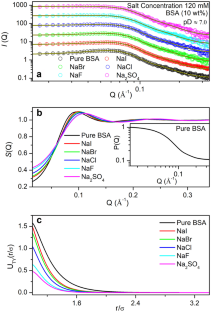
摘要用小角中子散射(SANS)技术研究了在阴离子(Cl -、Br -、I -、F -、SO42 -)存在的情况下,球状蛋白(牛血清白蛋白,BSA)在等电点(pI≈4.8)以上和以下的相互作用。SANS研究表明,在阴离子存在的情况下,BSA分子之间的短程吸引力几乎保持不变,而在Hofmeister系列阴离子存在后,中程排斥相互作用增加。虽然相互作用强度在BSA的pI以下和pI以上都有变化,但基本遵循这个级数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interaction among bovine serum albumin (BSA) molecules in the presence of anions: a small-angle neutron scattering study
Abstract
Protein–protein interaction in solution strongly depends on dissolved ions and solution pH. Interaction among globular protein (bovine serum albumin, BSA), above and below of its isoelectric point (pI ≈ 4.8), is studied in the presence of anions (Cl–, Br–, I–, F–, SO42–) using small-angle neutron scattering (SANS) technique. The SANS study reveals that the short-range attraction among BSA molecules remains nearly unchanged in the presence of anions, whereas the intermediate-range repulsive interaction increases following the Hofmeister series of anions. Although the interaction strength modifies below and above the pI of BSA, it nearly follows the series.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Biological Physics
生物-生物物理
CiteScore
3.00
自引率
5.60%
发文量
20
审稿时长
>12 weeks
期刊介绍:
Many physicists are turning their attention to domains that were not traditionally part of physics and are applying the sophisticated tools of theoretical, computational and experimental physics to investigate biological processes, systems and materials.
The Journal of Biological Physics provides a medium where this growing community of scientists can publish its results and discuss its aims and methods. It welcomes papers which use the tools of physics in an innovative way to study biological problems, as well as research aimed at providing a better understanding of the physical principles underlying biological processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


