高温质谱法研究GeO2的汽化热力学
IF 1.5
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
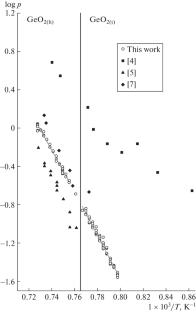
在1250 ~ 1370 K的温度范围内,用Knudsen渗液法结合质谱法研究了GeO2(四边形和六边形)的汽化。结果表明,GeO2上的饱和蒸汽由GeO和O2分子组成。已经确定了平衡蒸汽组分的分压及其与温度的关系。用第二定律和第三定律计算得到了非均相反应GeO2(s)的标准焓= GeO(g) + 1/2O2(g)和GeO2的标准生成焓(∆fH \(_{{298}}^{^\circ }\) (GeO(g)) = - 41.7±17.6 kJ mol-1)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Vaporization Thermodynamics of GeO2 by High-Temperature Mass Spectrometry
Vaporization of GeO2 (tetragonal and hexagonal) have been studied by the Knudsen effusion method in combination with mass spectrometry in the temperature range 1250–1370 K. It has been demonstrated that the saturated vapor over GeO2 consists of GeO and O2 molecules. Partial pressures of the equilibrium vapor components and their temperature dependences have been determined. The standard enthalpy of the heterogeneous reaction GeO2(s) = GeO(g) + 1/2O2(g) and the standard enthalpy of formation of GeO2 (∆fH\(_{{298}}^{^\circ }\)(GeO(g)) = −41.7 ± 17.6 kJ mol–1) have been determined by the second- and third-law calculations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Physical Chemistry
化学-物理化学
CiteScore
1.50
自引率
0.00%
发文量
9
审稿时长
6-12 weeks
期刊介绍:
Doklady Physical Chemistry is a monthly journal containing English translations of current Russian research in physical chemistry from the Physical Chemistry sections of the Doklady Akademii Nauk (Proceedings of the Russian Academy of Sciences). The journal publishes the most significant new research in physical chemistry being done in Russia, thus ensuring its scientific priority. Doklady Physical Chemistry presents short preliminary accounts of the application of the state-of-the-art physical chemistry ideas and methods to the study of organic and inorganic compounds and macromolecules; polymeric, inorganic and composite materials as well as corresponding processes. The journal is intended for scientists in all fields of chemistry and in interdisciplinary sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


