新型“HYDALJSS08”水醇多草药制剂的开发和超高效液相色谱分离、测定穿心莲全株中的穿心莲内酯和一种市场化的Siddha基多草药制剂“Kabusula Kudineer”
IF 1.5
4区 医学
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
以悉达达为基础的多草药配方被称为“Kabusura Kudineer”(已上市)并开发为“HYDALJSS08”水醇多草药配方,其中含有干燥原料形式的15种植物材料。由于其免疫增强特性,印度政府阿尤什部强烈建议在2019冠状病毒病大流行期间使用“Kabusura Kudineer”。本研究旨在扩大和验证穿心莲内酯(穿心莲内酯)的分析图谱,并从穿心莲全株和复方制剂(上市号:kabusura Kudineer, &开发号:HYDALJSS08)中分离穿心莲内酯(AP)。“Kabusura Kudineer”的有效成分之一是kalmeh,也被称为苦之王(Andrographis Paniculata-Acanthaceae)。Kalmegh含有穿心莲内酯(Andrographolides, AP)的主要活性成分,已被证明具有抗病毒和免疫调节活性。采用薄层色谱(TLC)和红外光谱(FT-IR)对AP和样品进行初步鉴别。液相色谱采用Zorbaz SB C8 (250*4.6mm & 5μm)。流动相为pH为2.8的磷酸缓冲液和乙腈:甲醇(60:30:10)。流动相流速为1ml/min,用紫外检测器在223 nm处观察流出物。色谱上运行时间为10 min,并观察保留时间。穿心莲内酯(AP)的Rf值为0.62。遵循ICH指南执行验证参数。AP的保留时间为2.5 min,有效参数为SD (1831.11), % RSD(0.2),回归方程为y = 41978 +x−10763,相关系数(R2) 0.9994。在5 ~ 50 μg/ml范围内建立良好的线性关系,检出限为0.61μg /ml,检出限为2.01 μg/ml,方法精密度% RSD为0.2,SD为1597.1,回收率为99.9%和101%。制剂“kaburiakudineer”中AP含量为1.48 μg/mL,开发的“HYDALJSS08”水醇复方制剂-0.48 μg/mL),从穿心莲中分离到穿心莲内酯112.4μg/ mL。所建立的高效液相色谱方法简便、新颖、快速、简便、准确、重现性好,可对分离的穿心莲内酯和悉达多药制剂进行线性分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
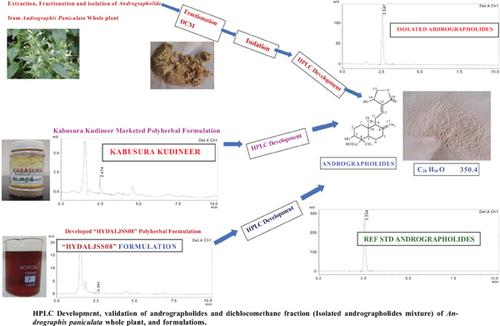
Novel “HYDALJSS08” Hydroalcoholic Polyherbal Formulation Development and Ultra-performance Liquid Chromatographic Separation,
Estimation of Andrographolides in Andrographis Paniculata whole Plants and a Marketed Siddha-based Polyherbal Formulation “Kabusura Kudineer”
The Siddha-based polyherbal formulation known as “Kabusura Kudineer (Marketed)"
and developed as “HYDALJSS08” hydroalcoholic polyherbal formulation contains some fifteen
plant materials in a dried raw form. Due to its immuno-booster properties, the Ministry of Ayush, Govt of
India, highly recommended the use of "Kabusura Kudineer" during the pandemic of COVID-19.
The present study intends to expand and validate the analytical profile for Andrographolides
(AP), and isolated Andrographolides (AP) from the Andrographis Paniculata whole plant
and in the Polyherbal Formulations (Marketed-Kabusura Kudineer, & Developed “HYDALJSS08”).
One of the active components of “Kabusura Kudineer” marketed and developed as
“HYDALJSS08” Hydroalcoholic Polyherbal formulation is kalmegh, also known as the king of bitter
(Andrographis Paniculata-Acanthaceae). Kalmegh composes active principal components of Andrographolides
(AP), which are proven for their Anti-viral and immunomodulatory activity. The preliminary
identification of AP and the sample was carried out by TLC and FT-IR. The liquid chromatography
was performed on a Zorbaz SB C8 (250*4.6mm & 5μm). The mobile phase incorporated pH 2.8
phosphate buffer with Acetonitrile: Methanol (60:30:10). The flow rate of the mobile phase was
1ml/min, and effluents were kept an eye on at 223 nm in a UV detector. The run time on the chromatogram
was 10 min, and retention time was also observed.
The Rf value of Andrographolides (AP) was found to be 0.62. ICH guidelines were followed
to carry out the Validation parameter. The retention time of AP was 2.5 min, and the Valid parameters of
AP and system precision were as follows: SD (1831.11), % RSD (0.2), regression equations y = 41978 +
x−10763, and correlation coefficient (R2) 0.9994. The adequate Linearity concentration was found to be
5 to 50 μg/ml, the value of LODs was 0.61μg /ml, LOQs was 2.01 μg/ml, method precision % RSD was
0.2, SD was 1597.1, and recovery was 99.9% and 101%. AP content found in a formulation (“Kabusura
Kudineer” 1.48 μg/mL, developed “HYDALJSS08” Hydroalcoholic Polyherbal formulation-0.48 μg/ml)
and isolated Andrographolides from Andrographis paniculata was 112.4μg/ml.
The developed HPLC methods enabled simple, novel, rapid, easy, accurate, reproducible,
and linear analysis of isolated andrographolides, and Siddha-based Polyherbal formulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.50
自引率
0.00%
发文量
85
审稿时长
3 months
期刊介绍:
Aims & Scope
Current Pharmaceutical Analysis publishes expert reviews and original research articles on all the most recent advances in pharmaceutical and biomedical analysis. All aspects of the field are represented including drug analysis, analytical methodology and instrumentation. The journal is essential to all involved in pharmaceutical, biochemical and clinical analysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


