对映选择性双模态交叉Diels-Alder反应及其在合成中的应用
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 6
摘要
与传统的 Diels-Alder 反应相比,两种不同共轭二烯之间的选择性交叉-Diels-Alder 反应,尤其是催化不对称方式的交叉-Diels-Alder 反应的发展一直被忽视。现在,我们报告了在铜(II)-双(噁唑啉)络合物催化下,3-烷氧基羰基-2-吡喃与未活化的共轭二烯发生的近对映和对映选择性交叉-Diels-Alder 反应,在温和的反应条件下产生了具有高对映选择性的正式反电子需求加合物。计算研究表明,该反应是通过一个模态过渡态进行的:过渡态后分岔导致具有相同对映体选择性的[2+4]和[4+2]加合物,然后进行简单的科普重排,提供单一的观察到的热力学[2+4]产物。该反应可与多种环戊二烯、富烯和环己二烯发生,为高密度官能化顺式双环支架提供了一种高效且具有对映选择性的方法。该反应的合成价值通过不对称合成两种具有重要生物学意义的天然产物青蒿酸和冠芥酸得到了证明。两种不同共轭二烯之间的全选择性催化不对称交叉-Diels-Alder 反应仍未得到充分发展。现在,我们克服了贫电子 2-pyrones 和未活化共轭二烯反应中的选择性难题,并确定了一种非模态过渡态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
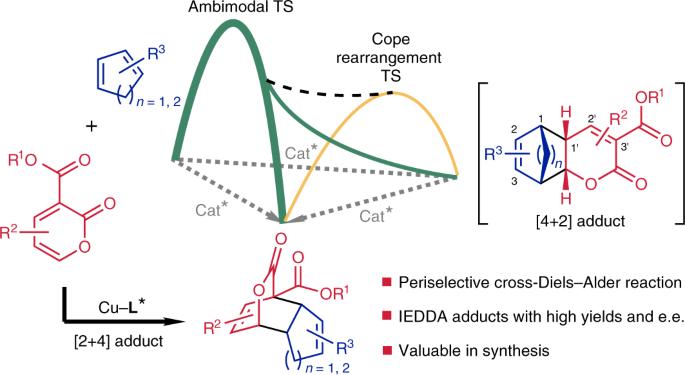
An enantioselective ambimodal cross-Diels–Alder reaction and applications in synthesis
Compared with the conventional Diels–Alder reaction, the development of selective cross-Diels–Alder reactions between two different conjugated dienes, especially in a catalytic asymmetric manner, has been neglected. We now report a peri- and enantioselective cross-Diels–Alder reaction of 3-alkoxycarbonyl-2-pyrones with unactivated conjugated dienes catalysed by a copper(II)–bis(oxazoline) complex, leading to formal inverse-electron-demand adducts with high enantioselectivity under mild reaction conditions. Computational studies showed that this reaction proceeds through an ambimodal transition state: post-transition-state bifurcation leads to [2+4] and [4+2] adducts with the same enantioselectivity, followed by a facile Cope rearrangement to provide a single observed thermodynamic [2+4] product. This reaction occurs with a wide variety of cyclopentadienes, fulvenes and cyclohexadienes, providing a highly efficient and enantioselective approach to densely functionalized cis-bicyclic scaffolds. The synthetic value of this reaction is demonstrated by the asymmetric synthesis of two biologically important natural products, artemisinic acid and coronafacic acid. Periselective catalytic asymmetric cross-Diels–Alder reactions between two different conjugated dienes remain underdeveloped. Now, the selectivity challenges are overcome in such a reaction of electron-poor 2-pyrones and unactivated conjugated dienes, and an ambimodal transition state is identified.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


