氨分解过程中亚胺锂表面的反应物诱导动力学
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于氨在可持续氢基经济中的潜在作用,人们对亚胺锂表面的氨分解进行了深入研究。在这里,我们通过先进的分子动力学模拟(ab initio accuracy)表明,催化剂的表面结构会在接触反应物时发生变化,并激活一种动态状态。正是这种高度波动的状态,而不是定义明确的静态催化中心,产生了催化作用。在这种活化的环境中,一系列最终导致释放出 N2 和 H2 分子的反应成为可能。一旦试剂流终止,亚胺表面就会恢复原始状态。我们认为,通过适当设计这种动态界面状态,可以设计出更好的催化系统。催化剂在转化过程中的常见静态描述经常受到质疑,但它们在这种条件下的具体性质仍然难以捉摸。现在,复杂的模拟揭示了氨在 LiNH 表面的分解是由一个高度动态的液态界面催化的,该界面在运行过程中可逆地形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
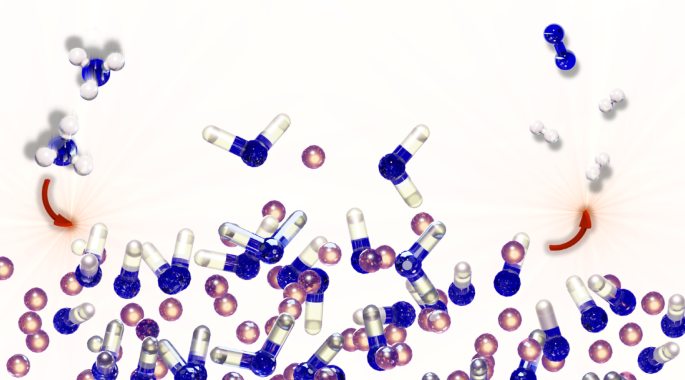
Reactant-induced dynamics of lithium imide surfaces during the ammonia decomposition process
Ammonia decomposition on lithium imide surfaces has been intensively investigated owing to its potential role in a sustainable hydrogen-based economy. Here, through advanced molecular dynamics simulations of ab initio accuracy, we show that the surface structure of the catalyst changes on exposure to the reactants and a dynamic state is activated. It is this highly fluctuating state that is responsible for catalysis and not a well-defined static catalytic centre. In this activated environment, a series of reactions that eventually leads to the release of N2 and H2 molecules becomes possible. Once the flow of reagent is terminated, the imide surface returns to its pristine state. We suggest that by properly engineering this dynamic interfacial state one can design improved catalytic systems. The common static description of catalysts during turnover has often been challenged, but their specific nature under such conditions remains elusive. Now complex simulations reveal that ammonia decomposition on LiNH surfaces is catalysed by a highly dynamic, liquid-like interface that reversibly forms under operation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


