氢辅助C-C偶联在氟改性铜上电催化还原CO2为乙烯和乙醇
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 496
摘要
电催化将二氧化碳还原成多碳(C2+)产物是一条极具吸引力的二氧化碳利用途径;然而,由于在高二氧化碳转化率下 C2+ 的选择性有限,C2+ 产物的产量仍然很低。我们在此报告了一种氟改性铜催化剂,该催化剂在流动池中电催化还原 CO2 时,电流密度高达 1.6 A cm-2,C2+(主要是乙烯和乙醇)法拉第效率为 80%。C2-4 选择性达到 85.8%,单程产率为 16.5%。我们展示了在吸附的 CHO 中间体之间形成 C2+ 的氢辅助 C-C 耦合机制。氟增强了水的活化、CO 的吸附以及吸附的 CO 与 CHO 中间体的氢化反应,而 CHO 中间体很容易发生偶联反应。我们的发现为设计具有实际应用潜力的高活性和选择性 CO2 电还原催化剂提供了机会。通过电催化将二氧化碳还原成多碳(C2+)产物是一条极具吸引力的二氧化碳利用途径。现在,一种氟改性铜催化剂在流动池中电催化还原二氧化碳时,电流密度达到 1.6 A cm-2,C2+ 法拉第效率达到 80%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
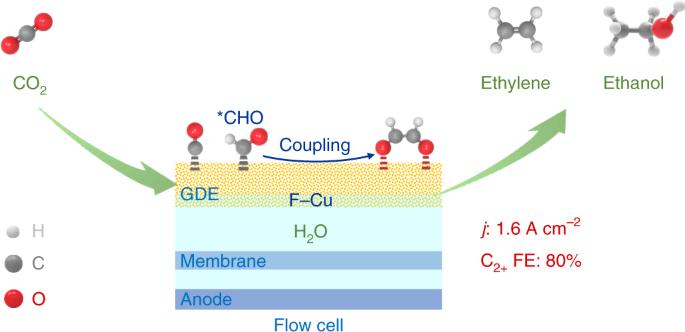
Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper
Electrocatalytic reduction of CO2 into multicarbon (C2+) products is a highly attractive route for CO2 utilization; however, the yield of C2+ products remains low because of the limited C2+ selectivity at high CO2 conversion rates. Here we report a fluorine-modified copper catalyst that exhibits an ultrahigh current density of 1.6 A cm−2 with a C2+ (mainly ethylene and ethanol) Faradaic efficiency of 80% for electrocatalytic CO2 reduction in a flow cell. The C2–4 selectivity reaches 85.8% at a single-pass yield of 16.5%. We show a hydrogen-assisted C–C coupling mechanism between adsorbed CHO intermediates for C2+ formation. Fluorine enhances water activation, CO adsorption and hydrogenation of adsorbed CO to CHO intermediate that can readily undergo coupling. Our findings offer an opportunity to design highly active and selective CO2 electroreduction catalysts with potential for practical application. Electrocatalytic reduction of CO2 into multicarbon (C2+) products is a highly attractive route for CO2 utilization. Now, a fluorine-modified copper catalyst is shown to achieve current densities of 1.6 A cm−2 with a C2+ Faradaic efficiency of 80% for electrocatalytic CO2 reduction in a flow cell.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


