高镁锂比盐湖盐水提锂研究进展
IF 15
2区 环境科学与生态学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 9
摘要
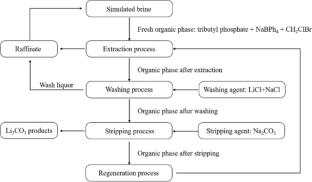
随着电动汽车、电池和电子设备的增加,对锂的需求正在迅速增长。锂可以从盐水中提取,但在高Mg/Li比下,锂离子Li+和镁离子Mg2+的分离是具有挑战性的。本文综述了萃取法、吸附法、纳滤法、选择性电渗析法和电化学插/脱插法等从盐水中提取锂的方法,重点介绍了高Mg/Li比下锂的选择性分离方法。萃取可以用有机溶剂和离子液体进行。用铝、锰和钛酸盐基吸附剂进行吸附。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lithium extraction from salt lake brines with high magnesium/lithium ratio: a review
The demand for lithium is growing rapidly with the increase in electric vehicles, batteries and electronic equipments. Lithium can be extracted from brines, yet the separation of lithium ions Li+ from magnesium ions Mg2+ is challenging at high Mg/Li ratios. Here, we review methods to extract lithium from brines, such as extraction, adsorption, nanofiltration, selective electrodialysis and electrochemical intercalation/de-intercalation, with focus on the selective separation of lithium at high Mg/Li ratios. Extraction can be done with organic solvents and ionic liquids. Adsorption is performed with aluminum-, manganese- and titanate-based adsorbents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Chemistry Letters
环境科学-工程:环境
CiteScore
32.00
自引率
7.00%
发文量
175
审稿时长
2 months
期刊介绍:
Environmental Chemistry Letters explores the intersections of geology, chemistry, physics, and biology. Published articles are of paramount importance to the examination of both natural and engineered environments. The journal features original and review articles of exceptional significance, encompassing topics such as the characterization of natural and impacted environments, the behavior, prevention, treatment, and control of mineral, organic, and radioactive pollutants. It also delves into interfacial studies involving diverse media like soil, sediment, water, air, organisms, and food. Additionally, the journal covers green chemistry, environmentally friendly synthetic pathways, alternative fuels, ecotoxicology, risk assessment, environmental processes and modeling, environmental technologies, remediation and control, and environmental analytical chemistry using biomolecular tools and tracers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


