一种用于药品质量控制的HPLC良好色谱规范诊断工具
IF 1.5
4区 医学
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
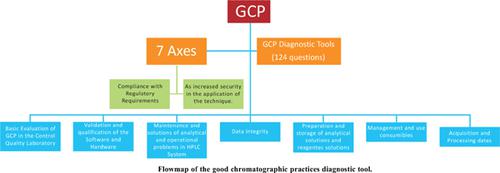
高效液相色谱法是制药行业质量控制中最常用的分析技术之一。由于这是一项复杂的技术,因此需要有助于遵守法规要求的良好做法。本研究旨在为质量控制实验室(QCL)的自我评估建立良好色谱规范(GCP)的诊断工具。这项研究是根据科学依据、制药立法以及制造商发布的指南进行的。确定了七个行动轴:实验室GCP的实施、管理和持续改进;设备和软件的安装、操作、鉴定和验证过程中的GCP;数据管理相关过程中的GCP,包括有关访问、生成、完整性和可追溯性的指南;与耗材管理和使用有关的GCP;与处理、维护、分析和操作故障排除相关的GCP;分析溶液和试剂溶液的制备、使用和储存过程中的GCP;以及与标准、样本和结果的获取和处理相关的GCP。这些轴产生了一个包含124个问题的诊断工具。GCP诊断工具的应用提供了与制药行业质量控制HPLC技术执行相关的常规和程序的映射,有助于满足监管要求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Diagnostic Tool for Good Chromatographic Practices Applied to HPLC in Pharmaceutical Quality Control
High-performance liquid chromatography is one of the most used analytical techniques in quality control in the pharmaceutical industry. Since it is a complex technique, it needs good practices that can contribute to compliance with regulatory requirements.
This study aims to establish a diagnostic tool for Good Chromatographic Practices (GCP) for the self-assessment of a Quality Control Laboratory (QCL).
The research was carried out on scientific bases, pharmaceutical legislation, as well as guides published by manufacturers.
Seven axes of action were identified: implementation, management, and continuous improvement of GCP in the laboratory; GCP in the installation, operationalization, qualification, and validation processes of the equipment and software; GCP in processes related to data management, including guidelines regarding access, generation, integrity, and traceability; GCP related to the management and use of consumables; GCP related to handling, maintenance, analytical and operational troubleshooting; GCP in the processes of preparation, use, and storage of analytical solutions and reagent solutions; and GCP related to the acquisition and processing of standards, samples, and results. These axes resulted in a diagnostic tool with 124 questions.
The application of the GCP diagnostic tool provides the mapping of the routine and procedures related to the execution of the HPLC technique for quality control in the pharmaceutical industry, contributing to meeting regulatory requirements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.50
自引率
0.00%
发文量
85
审稿时长
3 months
期刊介绍:
Aims & Scope
Current Pharmaceutical Analysis publishes expert reviews and original research articles on all the most recent advances in pharmaceutical and biomedical analysis. All aspects of the field are represented including drug analysis, analytical methodology and instrumentation. The journal is essential to all involved in pharmaceutical, biochemical and clinical analysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


