微孔水中氧还原反应活性增强
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 6
摘要
由可再生能源驱动的小气体分子电催化为碳中性燃料和化学品提供了一条前景广阔的途径。然而,由于气体分子在水中的溶解度较低,这种小分子转换反应依赖于水作为质子和电子的来源,从而限制了能量和功率密度。氧还原反应(ORR)就是这种限制的典范。在这里,我们证明了具有疏水内表面和亲水外表面的微孔纳米晶体所产生的多孔性水溶液的高载氧能力--微孔水--增强了水中的氧还原反应电催化。使用硅灰石-1 纳米晶体形成的高浓度 O2 微孔电解质溶液会大大增加 ORR 电流,以至于通常被认为是理想 ORR 催化剂的铂的活性受到部分限制,从而可以直接测量铂的内在催化 ORR 活性。涉及气体分子的电催化过程通常受到在水溶液中溶解度低的限制。在这里,利用硅灰石-1 纳米晶体赋予水以永久微孔来浓缩 O2,从而可以测量 Pt/C 催化剂在氧还原反应中的内在活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
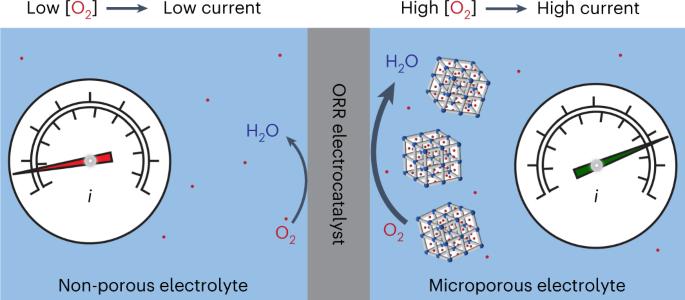
Enhanced activity for the oxygen reduction reaction in microporous water
Electrocatalysis of small gas molecules driven by renewable energy sources offers a promising route to carbon-neutral fuels and chemicals. Such small-molecule conversion reactions rely on water as a source of protons and electrons, however, thus limiting energy and power densities owing to the low solubility of gas molecules in water. The oxygen reduction reaction (ORR) is an exemplar of such limitations. Here we demonstrate that the high O2-carrying capacity of aqueous solutions endowed with porosity arising from microporous nanocrystals with hydrophobic internal surfaces and hydrophilic external surfaces—termed microporous water—enhances ORR electrocatalysis in water. Use of silicalite-1 nanocrystals to form an O2-concentrating microporous electrolyte solution increases the ORR current so much that the activity of Pt, typically thought to be an ideal ORR catalyst, is partially limiting, thus allowing the intrinsic catalytic ORR activity of Pt to be measured directly. Electrocatalytic processes involving gas molecules are generally limited by low solubility in aqueous solutions. Here water endowed with permanent microporosity by silicalite-1 nanocrystals is used to concentrate O2, allowing the measurement of the intrinsic activity of a Pt/C catalyst in the oxygen reduction reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


