电压门控Ca2+通道iq基序结合的CaBP1 c端结构域的化学位移赋值(CaV1.2)
IF 0.8
4区 生物学
Q4 BIOPHYSICS
引用次数: 0
摘要
神经元l型电压门控Ca2+通道(CaV1.2)与Ca2+结合蛋白1 (CaBP1)相互作用,促进Ca2+诱导的通道活性。CaBP1与CaV1.2中的iq基序的结合(残基1644-1665)阻断了钙调蛋白的结合并阻止了Ca2+依赖性CaV1.2的失活。Ca2+诱导的CaBP1与CaV1.2的结合对于调节神经元突触可塑性是重要的,这可能在学习和记忆中起作用。在这里,我们报告了CaBP1的c端结构域(残基99-167,称为CaBP1C)的NMR分配,该结构域包含两个Ca2+,结合在第三和第四个ef -手(EF3和EF4),并结合到CaV1.2 (BMRB登录号为CaV1.2)的CaV1.2 IQ-motif上。51518)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
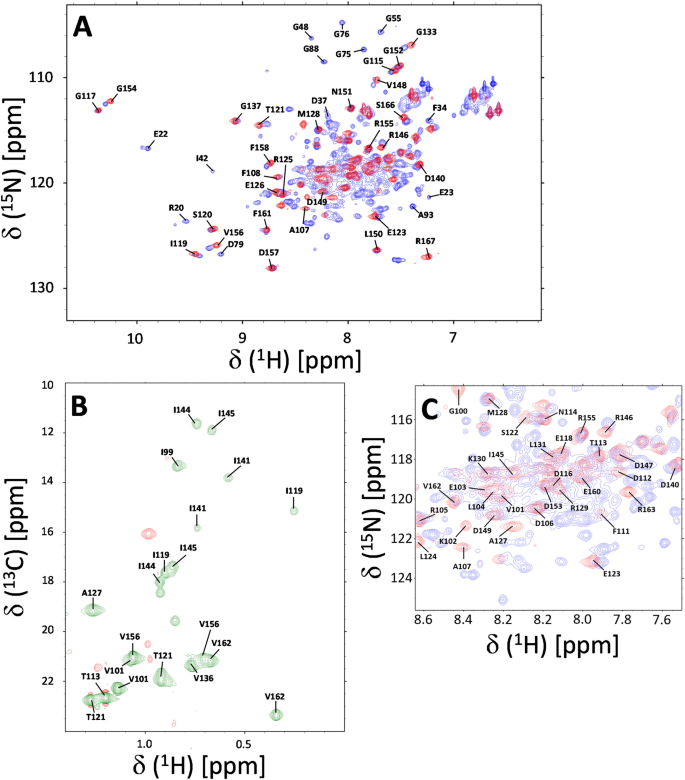
Chemical shift assignments of the C-terminal domain of CaBP1 bound to the IQ-motif of voltage-gated Ca2+ channel (CaV1.2)
The neuronal L-type voltage-gated Ca2+ channel (CaV1.2) interacts with Ca2+ binding protein 1 (CaBP1), that promotes Ca2+-induced channel activity. The binding of CaBP1 to the IQ-motif in CaV1.2 (residues 1644–1665) blocks the binding of calmodulin and prevents Ca2+-dependent inactivation of CaV1.2. This Ca2+-induced binding of CaBP1 to CaV1.2 is important for modulating neuronal synaptic plasticity, which may serve a role in learning and memory. Here we report NMR assignments of the C-terminal domain of CaBP1 (residues 99–167, called CaBP1C) that contains two Ca2+ bound at the third and fourth EF-hands (EF3 and EF4) and is bound to the CaV1.2 IQ-motif from CaV1.2 (BMRB accession no. 51518).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


