NMDA受体glun1c0结构域(残基841-865)上钙调蛋白的化学移位配位
IF 0.8
4区 生物学
Q4 BIOPHYSICS
引用次数: 2
摘要
大脑中的神经可塑性和突触传递由n -甲基- d -天冬氨酸受体(NMDARs)调节,NMDARs由甘氨酸结合GluN1和谷氨酸结合GluN2亚基的异四聚体组合组成。钙调蛋白(CaM)与GluN1的胞质C0结构域(残基841-865)结合,可能在NMDAR通道活性的Ca2+依赖性失活(CDI)中发挥作用。NMDARs的失调与各种神经系统疾病有关,包括阿尔茨海默病、抑郁症、中风、癫痫和精神分裂症。在这里,我们报告了Ca2+饱和CaM结合到人类NMDAR (BMRB no. 1)的GluN1 C0结构域的完整NMR化学位移分配。51715)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
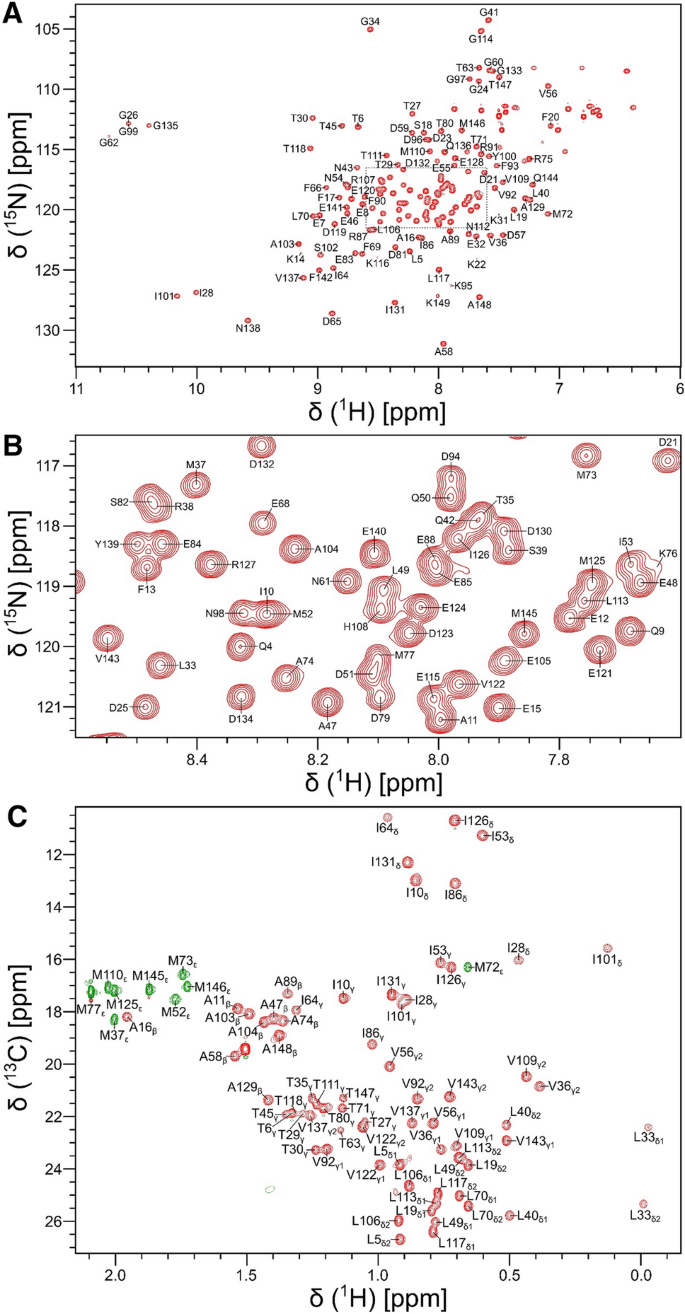
Chemical shift assignments of calmodulin bound to the GluN1 C0 domain (residues 841–865) of the NMDA receptor
Neuroplasticity and synaptic transmission in the brain are regulated by N-methyl-D-aspartate receptors (NMDARs) that consist of hetero-tetrameric combinations of the glycine-binding GluN1 and glutamate-binding GluN2 subunits. Calmodulin (CaM) binds to the cytosolic C0 domain of GluN1 (residues 841–865) that may play a role in the Ca2+-dependent inactivation (CDI) of NMDAR channel activity. Dysregulation of NMDARs are linked to various neurological disorders, including Alzheimer’s disease, depression, stroke, epilepsy, and schizophrenia. Here, we report complete NMR chemical shift assignments of Ca2+-saturated CaM bound to the GluN1 C0 domain of the human NMDAR (BMRB no. 51715).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


