双电层中氢键网络连通性主导了Pt电催化过程中pH的动力学效应
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 57
摘要
氢气电催化过程中存在较大的动力学 pH 值效应,即从酸性到碱性时反应动力学会下降约两个数量级,但这一现象的起源还远未达成共识。在这里,我们证明了是电双层中氢键网络的显著不同连通性导致了巨大的动力学 pH 效应。这一结果是通过细致地比较ab initio分子动力学模拟得出的酸性和碱性界面的电双层、ab initio分子动力学模拟得出的界面中水分子的振动态密度以及原位表面增强红外吸收光谱的结果而得出的。以铂-钌合金为模型催化剂,我们进一步揭示了羟基吸附在改善碱性氢气电催化动力学方面的意想不到的作用,即通过增加电双层中氢键网络的连通性,而不仅仅是影响表面反应步骤的能量。这些发现凸显了电双层结构在电催化中的关键作用。铂电催化剂上的氢进化和氧化反应在酸性电解质中比在碱性电解质中表现出更有利的动力学特性。现在,通过将理论模拟和光谱测量相结合,可以证明电双层中氢键网络的不同连通性是产生这种效应的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
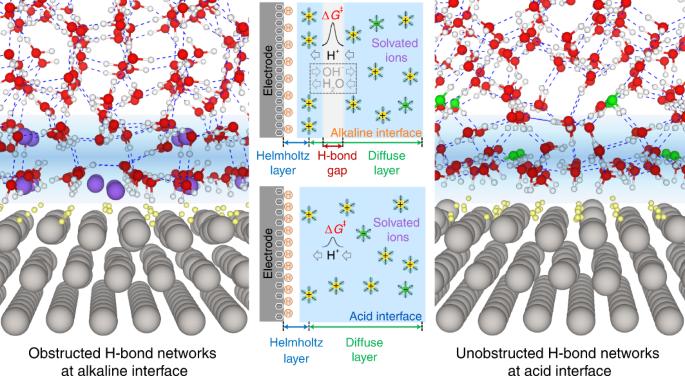
Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt
The origin of the large kinetic pH effect in hydrogen electrocatalysis, that is, the approximately two orders of magnitude decrease in reaction kinetics when moving from acid to alkaline, remains far from having a consensus. Here we show that it is the significantly different connectivity of hydrogen-bond networks in electric double layers that causes the large kinetic pH effect. This result has been obtained by meticulously comparing the electric double layers of acid and alkaline interfaces from ab initio molecular dynamics simulations, and the computed vibrational density of states of water molecules in the interfaces simulated with ab initio molecular dynamics, with the results of in situ surface-enhanced infrared absorption spectroscopy. Using a Pt–Ru alloy as a model catalyst, we further reveal an unanticipated role of OH adsorption in improving the kinetics of alkaline hydrogen electrocatalysis, namely, by increasing the connectivity of hydrogen-bond networks in electric double layers rather than by merely affecting the energetics of surface reaction steps. These findings highlight the key roles of electric double layer structures in electrocatalysis. The hydrogen evolution and oxidation reactions on Pt electrocatalysts exhibit much more favourable kinetics in acidic than in alkaline electrolytes. Now, by combining theoretical simulations and spectroscopic measurements, it is demonstrated that the different connectivity of hydrogen-bond networks in the electric double layer is responsible for such an effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


