解释B2H6的成键和3中心2电子键的性质:价理论的决定性检验
IF 1.8
3区 化学
Q1 HISTORY & PHILOSOPHY OF SCIENCE
引用次数: 1
摘要
在过去的一个世纪里,人们提出了许多关于B2H6成键的图片,从一开始,人们努力迫使B2H6符合目前C2H6的观点,到现在,人们把分子轨道模型看作是一个新的可转移概念的基础,这个概念是3中心-2电子成键,刺激了新化学的产生。即使是现在,也有人认为这个特殊的键更像是质子化的双键。这里总结一下目前对B2H6成键理解的历史发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
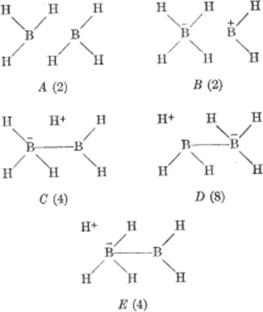
Interpreting the bonding of B2H6 and the nature of the 3-center-2-electron bond: decisive test of theory of valency
Many pictures of the bonding of B2H6 have been presented over the past century, starting with a strong effort to force B2H6 to fit the ideas that were current for C2H6 and building to now viewing the molecular orbital model as the basis for a new transferrable concept of a 3-center-2-electron bond that stimulates creation of new chemistry. Even now, though, some would view this special bond more like a protonated double bond. The historical development of the current understanding of bonding in B2H6 is summarized here.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Foundations of Chemistry
HISTORY & PHILOSOPHY OF SCIENCE-
自引率
22.20%
发文量
35
期刊介绍:
Foundations of Chemistry is an international journal which seeks to provide an interdisciplinary forum where chemists, biochemists, philosophers, historians, educators and sociologists with an interest in foundational issues can discuss conceptual and fundamental issues which relate to the `central science'' of chemistry. Such issues include the autonomous role of chemistry between physics and biology and the question of the reduction of chemistry to quantum mechanics. The journal will publish peer-reviewed academic articles on a wide range of subdisciplines, among others: chemical models, chemical language, metaphors, and theoretical terms; chemical evolution and artificial self-replication; industrial application, environmental concern, and the social and ethical aspects of chemistry''s professionalism; the nature of modeling and the role of instrumentation in chemistry; institutional studies and the nature of explanation in the chemical sciences; theoretical chemistry, molecular structure and chaos; the issue of realism; molecular biology, bio-inorganic chemistry; historical studies on ancient chemistry, medieval chemistry and alchemy; philosophical and historical articles; and material of a didactic nature relating to all topics in the chemical sciences. Foundations of Chemistry plans to feature special issues devoted to particular themes, and will contain book reviews and discussion notes. Audience: chemists, biochemists, philosophers, historians, chemical educators, sociologists, and other scientists with an interest in the foundational issues of science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


