Resmetrom治疗非酒精性脂肪肝:一项随机、双盲、安慰剂对照的3期试验。
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
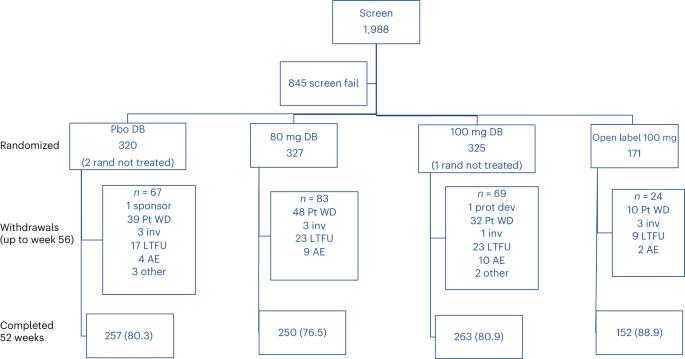
非酒精性脂肪性肝炎(NASH)是一种进展性肝病,尚无批准的治疗方法。MAESTRO-NAFLD-1是一项为期52周的随机、双盲、安慰剂对照的3期试验,评估雷司琼在患有非酒精性脂肪肝和假定NASH的成年人中的安全性。患者被随机分为三组(100 mg resmetrom(n = 325),80 mg resmetrom(n = 327)或安慰剂(n = 320))或开放标签100 mg resmetrom(n = 171)。主要终点是52周内治疗突发不良事件(TEAE)的发生率,关键的次要终点是LDL-C、apoB、甘油三酯(24周以上)、肝脂肪(16周和52周以上)和肝硬度(52周)。Resmetrom安全且耐受性良好。TEAE发生率为86.5%(开放标签100 mg雷司琼),86.1%(100 mg雷司琼),88.4%(80 mg resmetrom)和81.8%(安慰剂)的患者。超过安慰剂的TEAE包括治疗开始时的腹泻和恶心。关键次要终点包括80分时与安慰剂的最小二乘均数差异 mg,100 结果:低密度脂蛋白胆固醇(-11.1%,-12.6%),载脂蛋白B(-15.6%,-18.0%),甘油三酯(-15.4%,-20.4%),16周肝脂肪(-34.9%,-38.6%)(P本文章由计算机程序翻译,如有差异,请以英文原文为准。
Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial
Nonalcoholic steatohepatitis (NASH) is a progressive liver disease with no approved treatment. MAESTRO-NAFLD-1 was a 52-week randomized, double-blind, placebo-controlled phase 3 trial evaluating the safety of resmetirom in adults with nonalcoholic fatty liver disease and presumed NASH. Patients were randomized to three double-blind arms (100 mg resmetirom (n = 325), 80 mg resmetirom (n = 327) or placebo (n = 320)) or open-label 100 mg resmetirom (n = 171). The primary end point was incidence of treatment-emergent adverse events (TEAEs) over 52 weeks and key secondary end points were LDL-C, apoB, triglycerides (over 24 weeks), hepatic fat (over 16 and 52 weeks) and liver stiffness (over 52 weeks). Resmetirom was safe and well tolerated. TEAEs occurred in 86.5% (open-label 100 mg resmetirom), 86.1% (100 mg resmetirom), 88.4% (80 mg resmetirom) and 81.8% (placebo) of patients. TEAEs in excess of placebo included diarrhea and nausea at the initiation of treatment. Key secondary end points included least square means difference from placebo at 80 mg, 100 mg resmetirom: LDL-C (−11.1%, −12.6%), apoB (−15.6%, −18.0%), triglycerides (−15.4%, −20.4%), 16-week hepatic fat (−34.9%, −38.6%), (P < 0.0001) and liver stiffness (−1.02, −1.70) and 52-week hepatic fat (−28.8, −33.9). These findings demonstrate resmetirom was safe and well tolerated in adults with presumed NASH, supporting a role for further clinical development. (ClinicalTrials.gov identifier NCT04197479 ). In the phase 3 MAESTRO-NAFLD-1 trial, the liver-targeted thyroid hormone receptor-β selective agonist resmetirom was well tolerated and improved liver biomarkers in adults with nonalcoholic fatty liver disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


