癌症中的Stem样耗竭和记忆性CD8+T细胞。
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
T细胞在激活后可以获得广泛的分化状态。在这个连续体的极端是配备了效应器的短命细胞,以及更静止、更长寿的细胞,具有更高的增殖潜力和干细胞样的发育可塑性。后者包括干样耗竭T细胞和记忆T细胞,这两种细胞最近都已成为癌症免疫和免疫疗法反应的关键决定因素。在这里,我们讨论了干样耗尽的CD8+T细胞和记忆性CD8+T电池在调节和功能方面的关键相似性和差异性,并考虑了它们在癌症的不同结果中对保护性免疫的具体贡献,包括肿瘤逃逸、长期控制和根除。最后,我们强调了在理解干样耗竭T细胞和记忆T细胞的分子调节方面的最新进展是如何在癌症免疫疗法(如检查点抑制、过继细胞治疗和疫苗接种)中为临床益处而探索的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
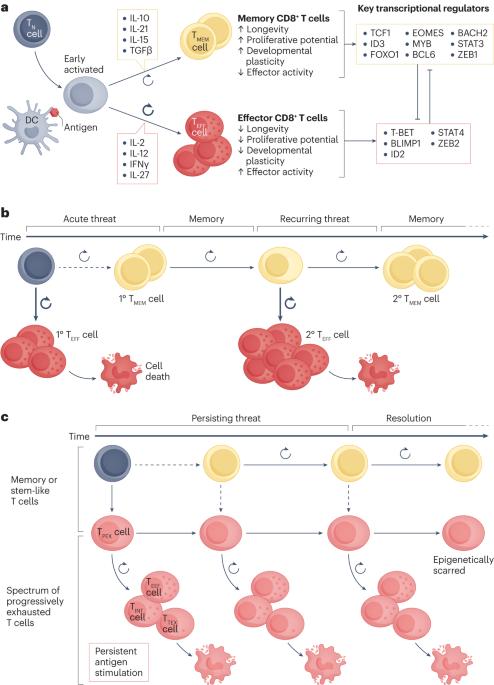
Stem-like exhausted and memory CD8+ T cells in cancer
T cells can acquire a broad spectrum of differentiation states following activation. At the extreme ends of this continuum are short-lived cells equipped with effector machinery and more quiescent, long-lived cells with heightened proliferative potential and stem cell-like developmental plasticity. The latter encompass stem-like exhausted T cells and memory T cells, both of which have recently emerged as key determinants of cancer immunity and response to immunotherapy. Here, we discuss key similarities and differences in the regulation and function of stem-like exhausted CD8+ T cells and memory CD8+ T cells, and consider their context-specific contributions to protective immunity in diverse outcomes of cancer, including tumour escape, long-term control and eradication. Finally, we emphasize how recent advances in the understanding of the molecular regulation of stem-like exhausted T cells and memory T cells are being explored for clinical benefit in cancer immunotherapies such as checkpoint inhibition, adoptive cell therapy and vaccination. T cells can acquire a broad spectrum of differentiation states following activation; certain subtypes of T cells have emerged as key determinants of cancer immunity and response to immunotherapies. Here, Gebhardt, Park and Parish discuss the phenotypic and functional variation of stem-like exhausted CD8+ T cells and memory CD8+ T cells, and how it contributes to their roles in immune escape and cancer outcome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


