使用胰岛素DNA折纸纳米结构激活多价胰岛素受体。
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
胰岛素结合胰岛素受体(IR)并调节靶组织中的合成代谢过程。IR信号受损与多种疾病有关,包括糖尿病、癌症和神经退行性疾病。据报道,即使在没有胰岛素结合的情况下,IRs也会在几种细胞类型的细胞膜上形成纳米簇。在这里,我们利用IR的纳米级空间组织来实现受控的多价受体激活。为了控制胰岛素纳米级的空间组织和价态,我们开发了杆状胰岛素DNA折纸纳米结构,该结构携带不同数量的胰岛素分子,具有特定的间距。增加每个纳米结构的胰岛素价显著延长了胰岛素DNA折纸纳米结构在受体处的停留时间。胰岛素价态和间隔都影响脂肪细胞中IR激活的水平。此外,与最高水平的IR激活相关的多价胰岛素设计也比相应的单价胰岛素纳米结构更有效地诱导胰岛素介导的转录反应。在糖尿病的体内斑马鱼模型中,用多价而非单价胰岛素纳米结构治疗导致葡萄糖水平降低。我们的结果表明,以纳米级精度控制胰岛素多价性和空间组织可以调节IR反应,而与胰岛素浓度无关。因此,我们建议将胰岛素纳米级组织作为开发新的胰岛素疗法的设计参数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
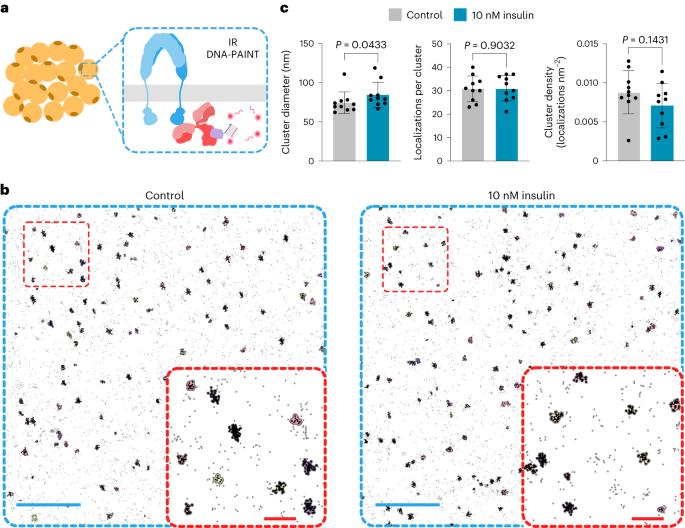
Multivalent insulin receptor activation using insulin–DNA origami nanostructures
Insulin binds the insulin receptor (IR) and regulates anabolic processes in target tissues. Impaired IR signalling is associated with multiple diseases, including diabetes, cancer and neurodegenerative disorders. IRs have been reported to form nanoclusters at the cell membrane in several cell types, even in the absence of insulin binding. Here we exploit the nanoscale spatial organization of the IR to achieve controlled multivalent receptor activation. To control insulin nanoscale spatial organization and valency, we developed rod-like insulin–DNA origami nanostructures carrying different numbers of insulin molecules with defined spacings. Increasing the insulin valency per nanostructure markedly extended the residence time of insulin–DNA origami nanostructures at the receptors. Both insulin valency and spacing affected the levels of IR activation in adipocytes. Moreover, the multivalent insulin design associated with the highest levels of IR activation also induced insulin-mediated transcriptional responses more effectively than the corresponding monovalent insulin nanostructures. In an in vivo zebrafish model of diabetes, treatment with multivalent—but not monovalent—insulin nanostructures elicited a reduction in glucose levels. Our results show that the control of insulin multivalency and spatial organization with nanoscale precision modulates the IR responses, independent of the insulin concentration. Therefore, we propose insulin nanoscale organization as a design parameter in developing new insulin therapies. DNA-origami-based insulin assembly into well-defined nanoclusters reveals that insulin valency and spatial organization modulate insulin receptor activation and downstream responses independent of ligand concentration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


