复分解方案在立体控制合成某些官能化β-氨基酯和氮杂杂环中的应用。
IF 7
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在这篇报道中,我们的目的是深入了解复分解协议(ROM、RCM、RCEYM、CM、RRM)在合成各种氮杂环框架中的应用。由于β-氨基酸在肽化学和药物设计中具有很高的生物学潜力和重要性,我们的目的是通过上述复分解策略重点介绍这类化合物的合成过程和转化,重点是选择性、立体控制、底物导向效应或官能团耐受性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
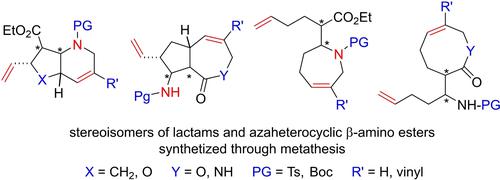
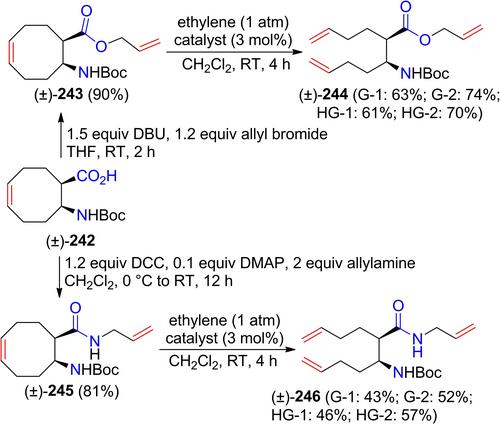
Application of Metathesis Protocols to the Stereocontrolled Synthesis of some Functionalized β-Amino Esters and Azaheterocycles
In this account our aim was to give an insight into the application of metathesis protocols (ROM, RCM, RCEYM, CM, RRM) for the synthesis of various azaheterocyclic frameworks. Due to the high biological potential and importance in peptide chemistry and drug design of β-amino acids our intention is to give a highlight on the synthetic procedures and transformation of these class of compounds with the above-mentioned metathesis strategies with emphasis on selectivity, stereocontrol, substrate-directing effect or functional group tolerance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical record
化学-化学综合
CiteScore
11.00
自引率
3.00%
发文量
188
审稿时长
>12 weeks
期刊介绍:
The Chemical Record (TCR) is a "highlights" journal publishing timely and critical overviews of new developments at the cutting edge of chemistry of interest to a wide audience of chemists (2013 journal impact factor: 5.577). The scope of published reviews includes all areas related to physical chemistry, analytical chemistry, inorganic chemistry, organic chemistry, polymer chemistry, materials chemistry, bioorganic chemistry, biochemistry, biotechnology and medicinal chemistry as well as interdisciplinary fields.
TCR provides carefully selected highlight papers by leading researchers that introduce the author''s own experimental and theoretical results in a framework designed to establish perspectives with earlier and contemporary work and provide a critical review of the present state of the subject. The articles are intended to present concise evaluations of current trends in chemistry research to help chemists gain useful insights into fields outside their specialization and provide experts with summaries of recent key developments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


